

Transformation stress resulting from the large volume changes accompanying solid-solid phase transformations can slow the rate of growth of the product phase by decreasing the thermodynamic driving force. Viscoelastic (plastic) relaxation of the elastic strain energy allows the reaction to proceed, at a rate controlled by the flow properties of the phases involved. Study of this phenomenon in high-pressure experiments therefore yields information not only on the effect of strain energy on growth kinetics but also on the rheology of high-pressure phases - which is difficult to study using conventional techniques. Previous experiments documented a cessation in growth at temperatures <= 1100°C during the olivine -> wadsleyite/ringwoodite transformation (see Section 3.1b in 1996 Annual Report). These earlier data are fitted well using a model for a misfitting spherical inclusion which assumes perfectly elastic behavior.
In order to investigate viscoelastic rheology of wadsleyite, new transformation
experiments are being performed on single crystal spheres of olivine embedded
in a NaCl pressure medium. The growth rates of wadsleyite rims, which nucleate
on the surface of the spheres and grow inwards to consume the olivine,
are determined as a function of time (Fig. 3.1-5).
 |
Fig 3.1-5: Optical photomicrograph (crossed nicols) showing a rim of wadsleyite which has grown to partly replace a spherical single crystal of San Carlos olivine during an experiment at 16 GPa and 1200°C for 5 minutes. |
This methodology offers several advantages over the previous study: (1) the growth process can be modelled accurately because of the spherical geometry (a near-cubic geometry was used in the previous study); (2) the use of NaCl as a pressure medium makes the external stress close to hydrostatic; (3) the lack of a fine-grained olivine matrix surrounding the single crystal (as in our previous study) eliminates possible drops in pressure caused by the volume reduction accompanying transformation of the matrix; and (4) the high energy of the NaCl-olivine interface promotes rapid nucleation, which minimises uncertainties in the determination of the growth rates.
In experiments at >= 17 GPa, intracrystalline
transformation occurs (see Section 3.1a), which complicates the reaction
mechanism and the determination of growth rates. Preliminary results
obtained at 16 GPa and 1100-1200°C show that rim growth slows but does
not cease (Fig. 3.1-6), suggesting that viscoelastic relaxation is taking
place on the time scale of these experiments. This is consistent with microstructures
observed optically and with TEM which indicate that recovery involving
dislocation climb in the wadsleyite rim occurs in the longer-duration/higher-temperature
experiments. The relatively high rate of relaxation in these new experiments
(compared with previous results) is due to the smaller sample size, which
reduces the capacity of the system to store elastic strain energy. Further
studies will be directed toward quantitative modelling the rim growth to
determine the rheologic behavior of wadsleyite and investigating the effects
of trace fluids. Small amounts of OH incorporated
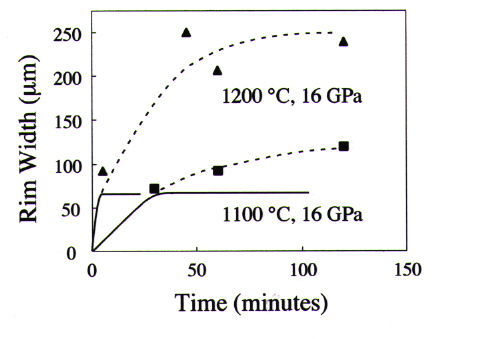 |
Fig. 3.1-6: Growth of wadsleyite rims as a function of time, showing that growth is non-linear, as expected when the growth rate is controlled by the rate of viscoelastic relaxation of elastic strain energy. The dashed lines show the approximate trend of the experimental data. The solid lines show the growth distance as a function of time as predicted by an elastic model - because there is no relaxation in this case, growth ceases at an early stage. |
into the structure of olivine may affect the kinetics of the transformation by changing the reaction mechanism, by enhancing diffusion across the interface, and/or by increasing the rate of stress relaxation.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page