

Silicate perovskite is the main phase of the lower mantle and thus, given
the volume of the lower mantle, the main phase of the Earth. Properties
of the perovskite phase determine the bulk physical and chemical properties
of the Earth. Recent experiments (Annual Report 1996) indicate that Al
can be incorporated in the perovskite structure. Al-bearing perovskites,
which are believed to be formed under lower mantle conditions, can be found
in nature as diamond inclusions. Examination of these synthesised and natural
perovskites demonstrates that incorporation of Al in the perovskite structure
affects the Fe3+/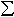 Fe ratio.
The presence of Fe3+ in the perovskite phase has effects on
electrostatic charge balance and equilibrium defect concentration. Electrical
conductivity, diffusivity characteristics and mechanical behaviour for
example, are sensitive to such changes.
Fe ratio.
The presence of Fe3+ in the perovskite phase has effects on
electrostatic charge balance and equilibrium defect concentration. Electrical
conductivity, diffusivity characteristics and mechanical behaviour for
example, are sensitive to such changes.
To investigate the effect of Al content on Fe3+/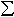 Fe ratio in perovskites, we synthesised samples in a multi-anvil press
at conditions relevant to the lower mantle (26 GPa, ~1700°C, runtime
2 h). By using two different capsule materials (iron and rhenium) we produced
relatively reducing and oxidising conditions respectively during the synthesis.
The samples were examined by X-ray diffraction, electron microprobe and
variable-temperature Mössbauer spectroscopy. Using Mössbauer
spectroscopy, which is sensitive to iron in its different oxidation states,
we have studied the concentration and site distribution of Fe3+
in the crystal structure. The minimal spatial resolution is about 50µm. A recently developed technique based on Electron Energy Loss Spectroscopy
(EELS) enables determination of Fe3+/
Fe ratio in perovskites, we synthesised samples in a multi-anvil press
at conditions relevant to the lower mantle (26 GPa, ~1700°C, runtime
2 h). By using two different capsule materials (iron and rhenium) we produced
relatively reducing and oxidising conditions respectively during the synthesis.
The samples were examined by X-ray diffraction, electron microprobe and
variable-temperature Mössbauer spectroscopy. Using Mössbauer
spectroscopy, which is sensitive to iron in its different oxidation states,
we have studied the concentration and site distribution of Fe3+
in the crystal structure. The minimal spatial resolution is about 50µm. A recently developed technique based on Electron Energy Loss Spectroscopy
(EELS) enables determination of Fe3+/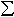 Fe with the transmission electron microscope. The results from the Mössbauer
experiments will be used to calibrate EELS-spectra as a complementary method,
allowing the determination of the Fe3+/
Fe with the transmission electron microscope. The results from the Mössbauer
experiments will be used to calibrate EELS-spectra as a complementary method,
allowing the determination of the Fe3+/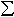 Fe ratio on a nanometer scale. EELS can also be performed on pervoskites
with low Fe concentrations.
Fe ratio on a nanometer scale. EELS can also be performed on pervoskites
with low Fe concentrations.
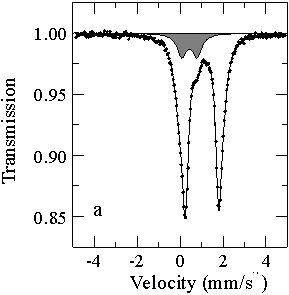
The Mössbauer spectra (Fig. 3.3-1) demonstrate that the presence of Al3+ in the perovskite allows significantly higher amounts of Fe3+ to be incorporated compared to the Al-free material. Further experiments shows that the influence of oxygen fugacity on this substitution is secondary. Therefore in the Earth´s lower mantle, which is believed to contain some 4 wt% Al2O3, the perovskite will contain highly oxidised Fe.
 |
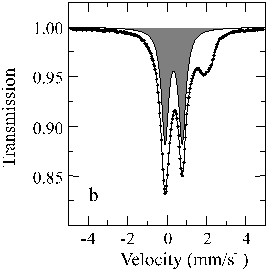 |
 Fe) is 12 % for the Al-free perovskite and 64 % for the perovskite containing
Al.
Fe) is 12 % for the Al-free perovskite and 64 % for the perovskite containing
Al. |
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page