

The atomic arrangement for AB2O4 type ternary compounds is known to depend on the relative sizes of A and B type cations; the radius ratio determines the stability of the relevant structure type. Although simple considerations based upon the cation sizes lead to the prediction that chromous orthosilicate should adapt the olivine structure, Cr2SiO4 was found to crystallize in the thenardite type (see Annual Report 1993), which is otherwise only known for larger B cations relative to the Si cation on the A site.
The chromium atoms in Cr2SiO4 are strongly displaced from the central position of the octahedral site relative to the positions in isotypic thenardite-type compounds. This leads to an unusual reduction of the Cr-Cr distance between adjacent polyhedra. The Cr-Cr distance (2.75Å) falls between the distances known for quadruple Cr-Cr bonds (1.90-2.55 Å) in binuclear [Cr2]2+ complexes and non-interactive metal-metal distances (>3.10 Å). This Cr-Cr distance suggests that there is significant electronic interaction or perhaps metal-metal bonding between the Cr atoms. Experimental work in the subsolidus equilibrium stability field of Cr2SiO4 has now enabled the growth of larger single crystals suitable for X-ray diffraction, spectroscopic and magnetic measurements to investigate this bonding further.
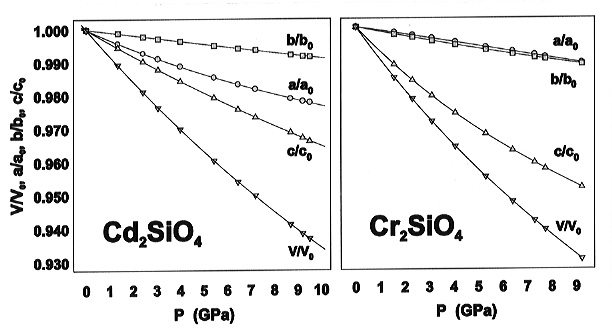
From high-pressure studies of the crystal structures of Cr2SiO4 and isotypic Cd2SiO4 we found distinct differences in the compressional behaviour what can be attributed to the different stereochemical requirements of bonding for the octahedrally coordinated M (=Cr, Cd) cations: (1) the bulk modulus of Cr2SiO4 (94.7 GPa) is smaller than that of Cd2SiO4 (119.2 GPa) although its molar volume (45.93 cm3 mol-1) is smaller than that of Cd2SiO4 (52.38 cm3 mol-1) this is in contrast to the general observation that the bulk modulus decreases with increasing molar volume in a series of isostructural compounds; (2) the compressional anisotropy of the unit cell is different reflecting the differences in compression of the MO6 octahedra (Fig. 3.3-2); (3) the pressure-induced displacements of the oxygen atoms leads to an increased planarity of the CrO4 configuration with a higher compressibility of the short Cr-Cr contact relative to those of the non-interactive Cr-Cr contacts.
 |
|
Polarized optical absorption spectra with E//c (the c axis is parallel to the vector of metal-metal interaction) reveal one strong absorption band with an unusually large half width (~5000 cm-1), which is typical of metal-metal electronic transfer. Moreover, the absorption for E//c was found to increase relative to the total absorption with increasing pressure suggesting an enhanced metal-metal electronic transfer. Clear evidence for interaction between the Cr atoms is given from the diamagnetic response measurements. Cr2SiO4 has an unmeasureable (<10-8 Am2) magnetic momentum compared to the typical paramagnetic response for an isolated square-planar high-spin d4 Cr2+ complex (e.g. 1.4 10-6 Am2 in gillespite-type CaCrSi4O10) indicating pairing of electrons. Furthermore, molecular orbital calculations reveal a sigificant orbital delocalization for the dZ2 orbitals indicative of direct Cr-Cr interaction. This explains the strong antiferromagnetic behaviour of the binuclear Cr-Cr system and finally provides clear evidence for a metal-metal bonding interaction and the presence of a binuclear [Cr2]4+ complex in Cr2SiO4, the first known in any silicate.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page