

Incommensurate structures are those in which the periodicity of either atomic ordering or atomic displacements does not match the periodicity of the underlying lattice. Incommensurate behaviour is normally observed either in a very limited temperature range close to a normal transition in a simple compound (the incommensurate structure in quartz is a good example), or in solid solutions such as the minerals plagioclase and mullite, where alternative available substitutional ordering schemes are effectively segregated within the incommensurate structure (leading to the reduction in configurational entropy and energy). The origin of the incommensurate structure in the melilite structures is unusual in that it does not readily fit into either of these categories.
In melilite the incommensurate phase appears to be due to the presence of a low-temperature structural instability of the structure what is related to the coordination requirements of the Ca ion within the structure. Unlike most structure types, which would under these circumstances undergo a simple displacive phase transition, in melilite there appears to be a genuine absence of any appropriate simple derivative phase either at the zone centre or at the Brillouin zone boundary; none of the possible derivative structures that can be derived through space group theory provide the necessary flexibility, particularly of the Si2O7 groups, to re-coordinate the Ca ion properly with oxygens at low temperature. Therefore an incommensurate phase is formed instead.
Further theoretical analysis in combination with results from X-ray diffraction allow the symmetries involved in the incommensurate phase to be identified. Existing X-ray structural data indicate that the mirror planes are lost from the space group of the high-symmetry polymorph (P-421m) and that the component structures within the modulation have P-4 and P21212 symmetries although it is obviously not possible to establish from theory alone which of these two structures is predominant. Mössbauer spectra of iron-doped åkermanites are consistent with the proposed two coexisting structurally distinct components that differ in site symmetry for the iron-bearing sites. The spectra can be used to establish the relative proportions of these components in the incommensurate domain structure.
Finally we note that, since there are four independent incommensurate
scattering vectors in melilite it is necessary to introduce a favourable
fourth order term in any Landau free energy expansion describing the transition.
Hence we may conclude that the incommensurate transformation in melilite
will show first order character with an entropy change dependent on the
relative magnitude of this fourth order Landau term. High-pressure single-crystal
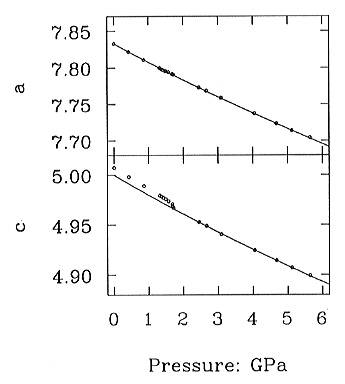
X-ray diffraction measurements show a smeared step in the pressure dependence
of the c-unit-cell parameter of Ca2MgSi2O7-melilite
(=åkermanite) at the transition (Fig. 3.3-3) and therefore
confirm the first-order nature of the incommensurate-normal transition.
A first-order component in the phase transition is also visible in pressure-dependent
Mössbauer spectra of Ca2Mg0.97Fe0.03Si2O7
åkermanite, collected up to 3.4 GPa. Upon increase of pressure (at
room temperature) the two quadrupole-split doublets first merge continuously
into each other (as they do with temperature increase) until a pressure
of 1.8± 0.2 GPa is reached where the
line width of the envelope suddenly drops to a value indicative of only
one Fe site.
 |
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page