

A high-pressure diffraction study of single crystals of CaSi2O5 (Annual Report 1996) shows that it undergoes a phase transition from a triclinic phase to a monoclinic phase at just 0.2 GPa at room temperature. The structure of the monoclinic phase was determined from single-crystal X-ray intensity data and is of the titanite aristotype, space group A2/a. Unlike the triclinic phase, which contains SiO5 polyhedra in addition to SiO4 tetrahedra and SiO6 octahedra, the monoclinic phase only contains tetrahedra and octahedra. The transition is zone-boundary, first-order in character with a 2.9% volume change.
The phase transition between the monoclinic and triclinic phases of
CaSi2O5 is the first direct observation of a displacive-like
transition involving a coordination change of silicon to be observed in
a crystalline oxide or silicate. Comparison of the two structures shows
that the overall topology and connectivity of the polyhedral framework
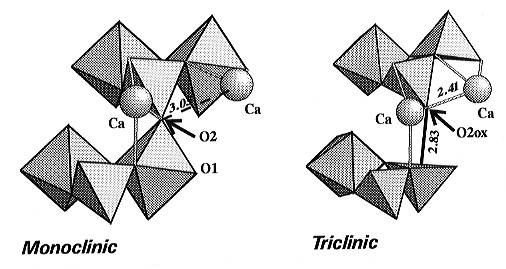
is maintained, with one major exception: one oxygen atom (denoted O2ox)
from every second SiO6 polyhedra in the octahedral chains of
the monoclinic phase is moved away to a non-bonded distance of 2.831(3)Å,
thereby breaking one eighth of the octahedral-tetrahedral bridges in the
structure. In order to maintain local charge balance at the displaced oxygen
the entire framework distorts and the Ca atoms are displaced significantly
so as to bring a second Ca atom into bonded contact with O2ox
(Fig. 3.3-4). Within the silicon-oxygen polyhedron that loses its sixth
oxygen at the transition the Si atom becomes displaced towards the oxygen
opposite that which is removed, and the coordination polyhedron becomes
a square-based pyramid (Fig. 3.3-4). All five of the remaining Si-O bond
distances become shorter.
 |
|
Silicon coordinated by five oxygen atoms appears to play a crucial role in determining transport properties in silicate melts at high pressures, diffusion rates in silicate perovskite, and has been implicated in the process of amorphisation of high-pressure silicates that often occurs upon pressure release. The mechanism of transformation of SiO6 octahedra into SiO5 groups has important consequences for the kinetics of these processes and the observations made on CaSi2O5 may be applicable to all of them. As in CaSi2O5 the driving force for the SiO6->SiO5 transformation will be the improvement in the local charge balance at the oxygen atoms in the structure.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page