

Water speciation measurements of volcanic glasses usually rely on the assumption that the glass retains the structure of the original melt, frozen in at the glass transition temperature. However, over the last few years, work performed mostly at the Geoinstitut has shown that this may be an invalid assumption and that there can be substantial changes in the water speciation below the glass transition in the structure. This can easily be studied using spectroscopic techniques, as the near infrared region of the spectrum of a hydrous glass has been shown to contain peaks reflecting the water species contained in the structure. This project is designed to increase our knowledge of such changes, by examining a series of rhyolitic glasses spectroscopically, and determining how the water speciation changes with both temperature and pressure.
A series of hydrous rhyolite glasses with water contents ranging from almost 0 to 5 wt% was synthesized using rapid-quench TZM-autoclaves. The total water content of each of the hydrous glasses was determined by Karl-Fischer titration and was found to be in good agreement with the amount of water originally weighed into the sample capsule. The homogeneity of the glasses was checked by infrared spectroscopy on various parts of the charge.
The water speciation can be determined using the peak found around 4500
cm-1, which is due to the combination stretching and bending
modes of X - OH groups (where X = Si, Al), and the peak around 5200 cm-1,
due to the combination stretching and bending modes of molecular H2O.
Spectra obtained from samples chosen from across the range of water contents
reveal that, in agreement with previous reported studies, all the glasses
containing >0.5 wt% H2Ototal contain detectable amounts
of molecular H2O. The spectra were used to determine the integral
extinction coefficients for the two peaks, revealing 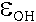 and
and 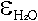 of 215.92 L mol-1cm-2
and 266.50 L mol-1 cm-2, respectively.
of 215.92 L mol-1cm-2
and 266.50 L mol-1 cm-2, respectively.
To investigate the effects of temperature on the water speciation, samples
of the glasses were placed on a heating stage, and spectra obtained at
temperature. In order to check that no water was lost during data collection
at elevated temperatures, the sample was allowed to cool to ambient conditions
after each heating experiment, and a room temperature spectrum was obtained.
This was checked against the original spectrum recorded at 25°C. Figure
3.5-7 shows
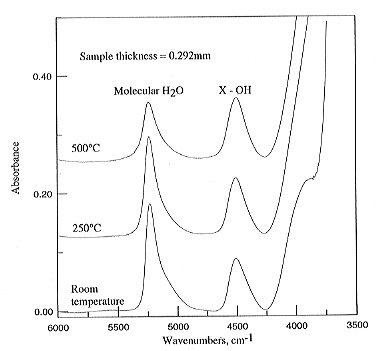 |
|
the evolution of the spectra obtained with increasing temperature for
a glass containing 4.94 wt% water. Apparently the absorbance AH2O
of the molecular water peak at 5200 cm-1 decreases with temperature,
while AOH at 4500
cm-1 increases. These effects could either be due to real speciation
changes or changes in extinction coefficients. In order to test for a possible
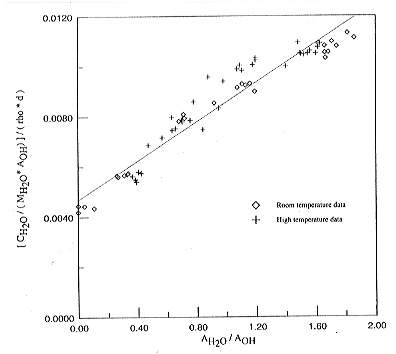
temperature dependence of extinction coefficients, Figure 3.5-8 shows a
plot of the ratio of cH2O / AOH
versus the ratio AH2O / AOH ,
where cH2O is the total water concentration. A linear regression
in this plot yields the extinction coefficients of OH and H2O
from the intercept at the y-axis and the slope of the curve. Inspection
of Fig. 3.5-8 shows that within experimental uncertainty, there is no significant
change of extinction coefficients with temperature. Accordingly, the changes
observed in the spectra must be due to real speciation changes. Using the
room temperature extinction coefficients, the concentrations of OH and
H2O in the samples can be calculated as a function of temperature
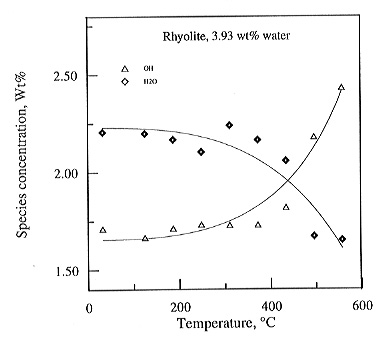
(Fig. 3.5-9). Clearly, there are massive changes above the glass transformation
around 400°C and possibly small changes already at lower temperature.
Experiments using externally heated diamond cells are currently under way
to study water speciation in rhyolite over a wide range of pressures and
temperatures. These experiments will also be extended to basaltic, andesitic
and phonolitic compositions.
 |
|
 |
|
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page