

Mg(OH)2 (brucite) sheets are important structural elements in many hydrous minerals such as chlorites, micas, and amphiboles. The crystal structure of brucite consists of a hexagonal closest-packed array of hydroxyl groups with Mg ions occupying octahedral interstices between alternate sheets. The O-H bonds are exclusively parallel to the c-axis. Due to this pronounced anisotropy and the high water content, brucite can be regarded as a model compound to study the effects of orientation, protons, and beam-induced dehydration on the O K-edge ELNES (Energy Loss Near Edge Structure) spectra. To study these effects, we have measured time- and orientation-dependent O K-edge ELNES spectra on two types of samples: (1) synthetic, chemically pure brucite powder and (2) natural single-crystal brucite from Pennsylvania, USA. The synthetic sample was used to monitor the process of dehydration, while the natural brucite was used to reveal the orientation-dependence and the effect of the presence of protons. For comparison, XANES/ELNES spectra were calculated on the basis of one-electron multiple scattering theory.
TEM observation of the synthetic sample reveals the immediate onset of decomposition under the electron beam, even when cooled to liquid nitrogen temperature (ca. 100 K). Dehydration starts at the rim of hexagonal brucite plates and proceeds towards the core, forming MgO with a porous microstructure. The dehydration is accompanied by distinct shrinkage of the plates (ca. 5% along the a- axis). In electron diffraction patterns, the originally sharp diffraction spots become diffuse and lens-shaped; the weak 10-10 reflections of brucite disappear during dehydration. The topotactic relationship between brucite and MgO revealed by electron diffraction is: [0001]Bru//[111]MgO and [11-20]]Bru//[1-10]MgO.
Two dehydration effects have been observed in a time-dependent series
of O K edge ELNES spectra measured on the synthetic
brucite sample (Fig. 3.8-11): (1) the broad (ca. 10 eV) onset maximum of
the fresh sample sharpens distinctly with increasing irradiation and the
following two peaks become more pronounced, and (2) the intensity of the
onset maximum increases as a function of time.
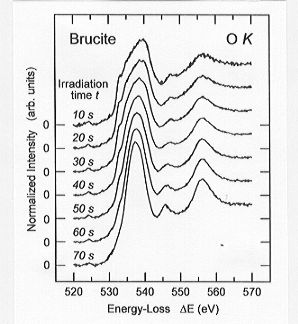 |
Fig. 3.8-11: A time series of O K edge ELNES spectra measured
on synthetic brucite powder. |
To optimize the orientation-dependent information in O K edge ELNES
spectra and to minimize irradiation damage, measurements on natural
single-crystal brucite were performed with a near parallel beam and low
collection angles. Under these conditions, the ratio of the momentum
transfer with q parallel and perpendicular to the beam was 4:1. A
comparison of the O K edge ELNES spectra with
momentum transfer parallel (q // c) and perpendicular (q  c) to the crystallographic c-axis reveals distinct differences in
terms of peak number and relative peak intensities (Fig. 3.8-12). The most
remarkable difference is the presence of a peak (F) in the q // c spectrum,
which, in turn, is absent in the q
c) to the crystallographic c-axis reveals distinct differences in
terms of peak number and relative peak intensities (Fig. 3.8-12). The most
remarkable difference is the presence of a peak (F) in the q // c spectrum,
which, in turn, is absent in the q  c
spectrum. Considering that the momentum transfer was not completely parallel
to the beam, the experimental O K edge spectra of brucite agree
well with those calculated by MS techniques (Fig. 3.8-12).
c
spectrum. Considering that the momentum transfer was not completely parallel
to the beam, the experimental O K edge spectra of brucite agree
well with those calculated by MS techniques (Fig. 3.8-12).
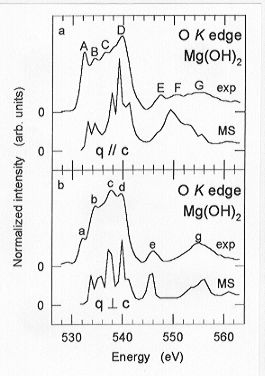 |
Fig. 3.8-12: Comparison of experimental and theoretical
O K edge ELNES spectra with momentum transfer parallel (q // c) and perpendicular
(q 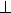 c) to the c-axis of single-crystal brucite. c) to the c-axis of single-crystal brucite. |
Since the O-H bonds are exclusively parallel to the c-axis the contribution
of protons must be in the q // c spectrum. The main differences between
the q // c and q 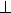 c spectra are intensity differences
in the broad onset maximum and the presence of an extra peak (F) in the
q // c spectrum. Dehydration causes distinct intensity and peak shape changes
at these energy positions, confirming the contribution of protons. Density
of state calculations also confirm the interpretation that peaks (D) and
(F) reflect the contributions of protons to the q // c spectrum.
c spectra are intensity differences
in the broad onset maximum and the presence of an extra peak (F) in the
q // c spectrum. Dehydration causes distinct intensity and peak shape changes
at these energy positions, confirming the contribution of protons. Density
of state calculations also confirm the interpretation that peaks (D) and
(F) reflect the contributions of protons to the q // c spectrum.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page