A major area of research in geochemistry is to understand how the Earth accreted and how the core segregated. Laboratory experiments to determine silicate-metal partitioning of highly siderophile elements (HSE), such as Pt, Rh, Re, Os and Ir, can yield valuable information on this problem. HSE, which should partition preferentially into a metallic phase, are overabundant in the mantle compared with the situation expected if the mantle equilibrated with bodies of segregating metallic core. The "late veneer theory" has been suggested to explain this anomaly: after core segregation and before mantle differentiation, a late veneer of extraterrestrial material (C1 chondrite) enriched the mantle in the HSE. The experimental work described here is being performed in order to test this model, by examining the effects of temperature, pressure, composition and oxygen fugacity (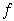 o2), on partitioning of HSE between metal and silicate melt.
o2), on partitioning of HSE between metal and silicate melt.
Osmium is one HSE that to date has been poorly investigated, mainly due to experimental problems such as its high volatility at low temperature and the very low solubility in silicate melt. In previous studies a large scatter in the experimental measurements was observed for samples equilibrated at very reducing conditions. This is probably due to the presence of micro-nuggets, or metal clusters that can dramatically effect the analysed concentration of osmium in the melt.
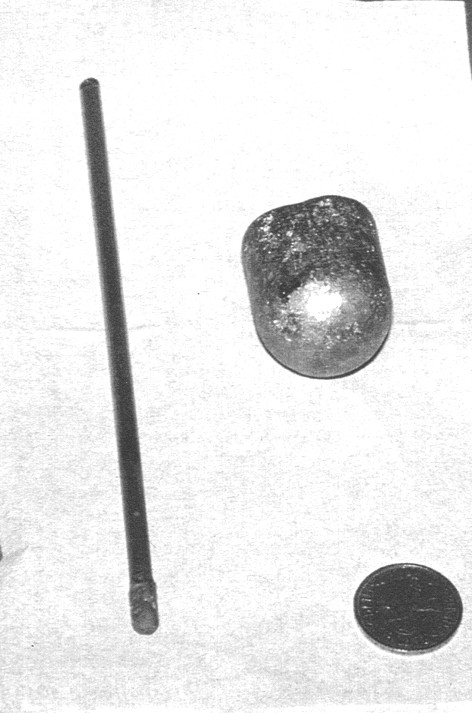
In order to improve on these results, a study of the effect of oxygen fugacity on the partitioning of osmium between metal and silicate melt has been performed using the mechanically assisted equilibration technique. A crucible of the metal of interest is filled with glass chips and heated in a vertical furnace, through which a CO:CO2 mixture is passed to control the 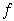 o2. A spindle of the same metal is then used to stir the melt. For this experiment, a crucible and a spindle made of Ni-Os alloy (5 wt% Os) were produced, as shown in Figure 3.3-1. The advantage of this technique is that the stirring sets up a convective regime
o2. A spindle of the same metal is then used to stir the melt. For this experiment, a crucible and a spindle made of Ni-Os alloy (5 wt% Os) were produced, as shown in Figure 3.3-1. The advantage of this technique is that the stirring sets up a convective regime
|
Fig. 3.3-1: Ni-Os alloy crucible and spindle. The spindle is composed of a pure Ni rod 150 mm long, 5 mm in diameter welded to the 10 mm long 5 mm diameter Ni-Os head. The crucible is 35 mm high, 35 mm outer diameter and 25 mm inner diameter. This crucible can contain approximately 30 mg of melt. |
in the liquid and any micro-nuggets should be swept to the walls, yielding a large amount of sample (500 mg) free of micro-nuggets. Sampling can be performed without interrupting the experiment by dipping an alumina rod into the melt, withdrawing it, and then quenching the attached melt in cold water. Time series data can be collected to prove that the equilibrium conditions were reached for each 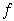 o2. Samples were analysed by negative thermal ionization mass spectroscopy (NTIMS) at IPG, Paris.
o2. Samples were analysed by negative thermal ionization mass spectroscopy (NTIMS) at IPG, Paris.
Figures 3.3-2 and 3.3-3 show the evolution with time of the osmium solubility in an anorthite/diopside eutectic melt at log 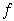 o2 = -12.3 and log
o2 = -12.3 and log 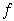 o2 = -11.2 respectively. The higher than expected initial concentration of osmium in the melt is attributed to the presence of dissolved oxygen in the starting material, which was synthesised in air. This oxygen reacts with the osmium, making it highly soluble in the melt. The data show that equilibrium should be reached after about 6 weeks.
o2 = -11.2 respectively. The higher than expected initial concentration of osmium in the melt is attributed to the presence of dissolved oxygen in the starting material, which was synthesised in air. This oxygen reacts with the osmium, making it highly soluble in the melt. The data show that equilibrium should be reached after about 6 weeks.
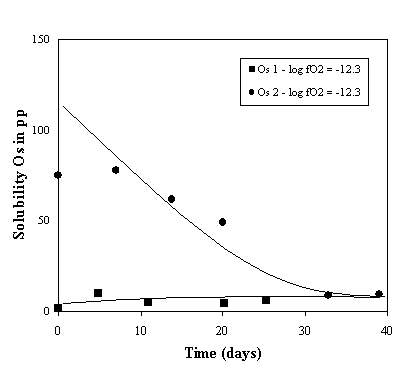 |
Fig. 3.3-2: Solubility of osmium in an anorthite diopside eutectic melt composition, T = 1350°C, log 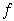 o2 = -12.3; analysis of the glass samples were performed by NTIMS.
o2 = -12.3; analysis of the glass samples were performed by NTIMS. |
Figure 3.3-4 compares our data with previously published results. The low fO2 data are comparable, though we suspect that micro-nuggets of osmium are still present in our samples and affect the concentrations obtained by NTIMS analysis. In order to improve on this, laser ablation ICPMS will be performed on these samples to determine the Os concentration. With this technique very small areas of the glasses can be analysed and thus the contamination due to micro-nuggets can be avoided.
 |
Fig. 3.3-3: Solubility of osmium in an anorthite diopside eutectic melt composition: T = 1350°C, log 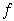 o2 = -11.2 analysis of the glass samples were performed by NTIMS.
o2 = -11.2 analysis of the glass samples were performed by NTIMS. |
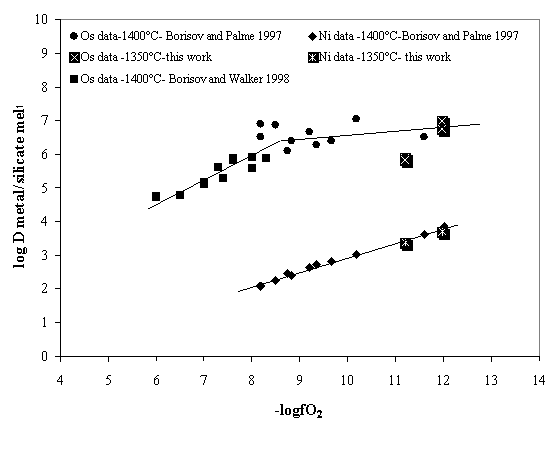 |
Fig. 3.3-4: Effect of oxygen fugacity on the partition coefficient of osmium between metal and an anorthite-diopside eutectic melt. Comparison of our data with those of Borisov & Palme (1997) and Borisov & Walker (1998) is shown. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page