

The sulfur content of mantle peridotites is controversally discussed to be either 20 ppm(wt) or 200 - 300 ppm(wt). As basaltic melts typically have sulfur contents between 500 and 1500 ppm(wt) and because sulfur is hardly soluble in silicates and oxides involved in the melting process, a sulfide phase needs to be present in the source regions of basaltic melts. This means that after the formation of the Earth's core some residual sulfide remained in the mantle. Peridotite xenoliths and peridotite massifs indeed contain sulfide phases, but these are re-equilibrated or altered and do not contain information on the nature of the sulfides under pressure and temperature conditions of the upper mantle. Questions thus arising, which are being investigated in this study, concern the conditions under which sulfide melts segregated from the mantle during core formation and how such conditions influence the behaviour of sulfide melts so that they can segregate.
Experimental investigations show that under mantle pressures and temperatures sulfides can exist as melts. Melts located at triple grain boundary junctions in crystalline aggregates may have either wetting or non-wetting behaviour. Wetting melts can segregate along grain boundaries when their volume exceeds about 0.1 vol%, whereas non-wetting melts are trapped at triple grain boundary junctions as ellipsoidal melt pockets. Pure iron-nickel sulfide melts are non-wetting. However, from experiments under different pressure-temperature conditions reported in the literature it is known that sulfide melts may contain up to several wt% oxygen. Therefore there might be a critical composition, depending on oxygen and sulfur fugacity, where iron-nickel-sulfur-oxygen melts change their behaviour from non-wetting to wetting. Also, sulfide melts are supposed to have a very low viscosity and might therefore segregate easily. As different oxygen and sulfur fugacities exist in different regions of the mantle, varying degrees of segregation may also be a possible explanation for the different observed sulfur contents in mantle peridotites.
The aim of this project is to investigate the composition and the wetting behaviour of Fe-Ni-S-O melts coexisting with silicate melts as a function of oxygen fugacity in ultramafic rocks. As a new experimental approach the oxygen fugacities were controlled by equilibria between olivine and ferrite-chromite spinel solid solutions, whereas the sulfur fugacity was not controlled. The experiments were performed in the system MgO-SiO2-Fe-Ni-Cr-S-O at 1400 °C and 2 GPa using San Carlos single crystal capsules welded into platinum outer capsules. The starting material consisted of a mechanical mixture of San Carlos olivine, synthetic orthopyroxene, synthetic ferrite-chromite spinel and FeS-NiS-FeO mixtures.
Both a Fe-Ni-S melt and a S-poor silicate melt were present in the experimental run products. In systems with magnetite the amount of silicate melt was higher than in systems with ferrite-chromite spinel. This may be the result of increasing solidus temperature with increasing Cr content of the sample. The Fe-Ni-S melt occurs as circular or ellipsoid inclusions in olivine or in contact with the silicate melt (see Fig. 3.2-5). The Fe-Ni-S melt is characteristically surrounded by a cavity that may be a shrinkage phenomenon resulting from cooling, or may be caused by an exsolved fluid formed during the quench. In some runs the S-poor silicate melt quenched to a glass containing small exsolved droplets of Ni-poor sulfide melt, whereas in other experiments, quench products also include olivine, orthopyroxene, and a quench silicate melt. Despite the wide range of oxygen fugacities covered, neither a change in the wetting behaviour of the Fe-Ni sulfide melts could be detected nor was any oxygen detected. The lack of oxygen might be due to unmixing because of a "sulfide-sulfate" solvus or to rapid exchange of oxygen with surrounding phases during the quench. In some runs orthopyroxene and Ni-poor sulfide were found in narrow cracks up to several hundred microns away from the sample cavity of the San Carlos olivine capsule. This indicates the existence of a SiO2-, Ni- and S-bearing fluid phase during the experiment.
The question if there occurs a change in the wetting behaviour of oxygen-bearing
sulfide melts is still open, and further experiments are in progress to
solve the problems discussed above.
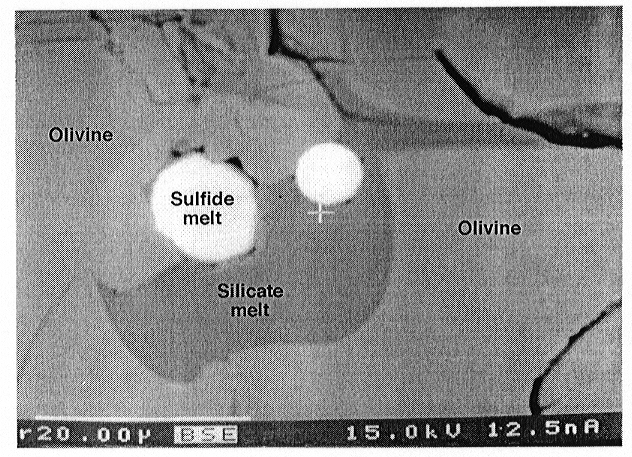 |
Fig. 3.2-5: Back scattered electron image of olivine (light grey) containing channels of wetting S-poor silicate melt (darker grey) and of non-wetting Fe-Ni sulfide melt droplets (white). Note that both Fe-Ni sulfide droplets show a narrow rim that could be the result of shrinkage or an exsolved fluid phase during quench. Scale bar is 20 µm. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page