

The electronic high-spin (t2g)3(eg)1 configuration of divalent chromium atoms and the resulting Jahn-Teller distortion make the crystal chemistry of Cr2+-containing silicate phases of interest for understanding the chemistry of the Earth's interior. Cr2+ silicates are known only for a few compounds such as olivine and pyroxene solid-solutions, the thenardite-type Cr2SiO4 and the gillespite-type CaCrSi4O10 compounds. The two new BaCrSi4O10 and SrCrSi4O10 phases, analogous to the existing ACuSi4O10 (A = Ca, Sr, Ba) series, were synthesized as part of ongoing investigations into the influence of the Jahn-Teller cation on the high-pressure phase transition which occurs in gillespite (BaFeSi4O10).
Synthesis experiments in the respective MeO-SiO2-Cr2O3-Cr0 systems were performed in evacuated quartz-glass tubes using excess chromium metal to keep fO2 below the Cr2O3-Cr0 buffer. Subhedral platy single crystals of pink to salmon-red colour and up to 150µm in size were grown from Li2B4O7 and Na2B4O7 fluxes in the temperature range 800° - 1020 °C at P<10-3 bar. Solid state reactions at 1350° - 1450 °C, without additional fluxes, yielded almost single-phase products of the Cr2+ compounds.
Crystal structure investigations by X-ray diffraction studies confirmed
that both BaCrSi4O10 and SrCrSi4O10
possess the tetragonal ABSi4O10 gillespite-type crystal
structure. Lattice parameters of the ACrSi4O10 series
are shifted towards slightly smaller values compared to those of the equivalent
Cu series (Fig. 3.3-4) owing to the smaller ionic radius of Cr2+.
The
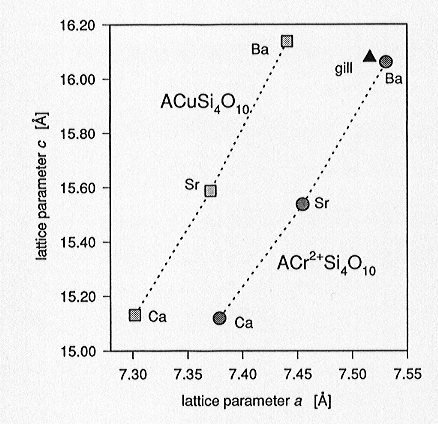 |
Fig. 3.3-4: Lattice parameters a, c for the synthesized ACr2+Si4O10 and ACuSi4O10 phases (A= Ba, Sr, Ca; gill= gillespite, BaFe2+Si4O10) |
predominating changes in a, induced by the substitution of Cr2+ for Cu2+, arise from the alignment of the square-planar CrO4 configurations parallel to (001). The <Cr[4pl]-O3> bond distances are 1.992(5) and 1.995(5) Å, which are in close agreement with the expected bond length of 1.986 Å for ideally 4-coordinated Cr2+ as derived from bond-valence calculations.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page