

Laboratory experiments on geological materials and analogues yield fundamental properties of minerals and fluids as well as information about how such phases interact. These properties then enable us to decipher the "experiments" that have taken place in the natural world where we only have the end product and no lab notebook with run duration, heating/cooling rate, compression/decompression rate, fluid composition, oxygen fugacity, starting composition and starting state noted. The goal of the metamorphic petrologist is to reconstruct the series of conditions that the studied rock samples endured as well as the time scale of the event (or multiple events) in order to gain insight into, and put constraints on, orogenic processes. Mathematical modeling of orogenic processes can provide a framework of possible timescales and pressure-temperature-time (P-T-t) trajectories, but how do these compare to what the rocks themselves deliver?
Of particular value in reconstructing P-T-t paths are rocks with multiple partial overprints where the sequence of partial assemblages can be equated to those expected from petrogenetic grids (a series of mineral stability zones and reaction boundaries in P-T space) to provide the broad pattern of P-T conditions experienced. Unfortunately, the reaction "snap-shots" alone do not reveal if the recorded reaction history represents a single heating-cooling cycle, multiple rejuvenations within a single cycle or even if it is the product of separate orogenic events of different age. Even if the different assemblages have been dated isotopically there is still no constraint on the actual temperature-time path between the reaction domains: this can only be estimated by considering the effects of a temperature-time-dependent process such as diffusion. Without the true P-T-t path, direct comparison with modelled paths may seriously misrepresent the actual tectonic processes experienced.
The studied sample, an eclogite (P>15 kbar, T ~
700 °C) from the Variscan orogenic belt (Bohemian Massif, Lower Austria),
which suffered a granulite facies overprint (P ~
7 kbar, T ~ 750 °C) during exhumation. Orthopyroxene
+ plagioclase has formed inside fractures in garnet due to reaction between
garnet and a quartz inclusion. If it is assumed that the absolute rim composition
of garnet next to the opx-plag zone is in equilibrium with the new assemblage,
then it would be expected that compositional differences in garnet away
from these rims would gradually decrease in time due to diffusion, i.e.
the new rim composition would, by diffusion, penetrate into the garnet
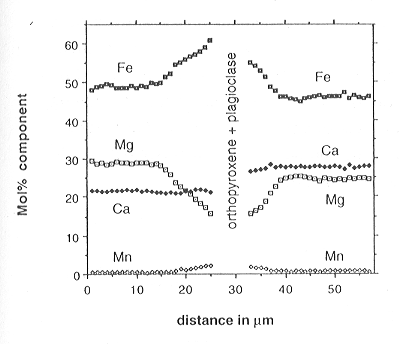
interior. An example of zoning in garnet on either side of such an opx-plag
fracture zone is shown in Fig. 3.5-1. The fracture happens to lie along
the boundary between different overgrowth zones - a position commonly marked
by numerous quartz inclusions - and so the flattened parts of the garnet
profile (the pre-opx compositions) are different on each side. The penetration
depths of the profiles are on the order of 10 µm, but as the absolute
rim cannot be reached by microprobe, the profile could be up to 2 µm
longer: a conservative estimate of the penetration depth for modeling was
taken to be 15 µm. If the garnet rim composition is controlled by
the assemblage in the fracture, then the diffusion problem can be tackled
as one of diffusion into an infinite sheet from a source of fixed composition.
The solution for the penetration depth of a profile by this model is 4√
Dt where t is time and D the diffusivity at the relevant temperature. The
time taken to generate a 15 µm profile at different temperatures
and for different garnet diffusivities is shown in Fig. 3.5-2. From all
reasonable garnet-orthopyroxene geothermometers (i.e. those designed for
Fe-rich compositions!) the temperature for the fracture assemblage is always
above 700 °C, as would be expected for a granulite facies assemblage.
Therefore, utilising Fig. 3.5-2, it is obvious that at 700 °C, regardless
of the diffusion data used (both sets are regarded as the slowest available
for garnet) the recorded degree of diffusion would have formed in less
than 100,000 years. Even if 700 °C was reached as the peak of a heating
followed by cooling path, the lower effective temperature for the whole
of this T-t path (so-called characteristic temperature) in this type of
isothermal diffusion model would only be reduced to 640 °C even for
contact metamorphic style heating and cooling scenarios.
 |
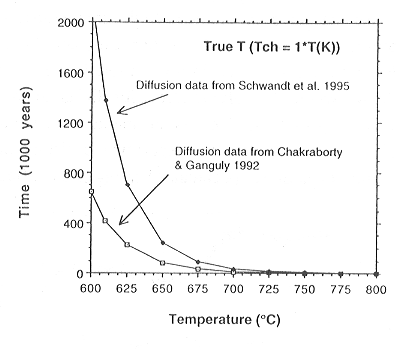 |
Fig. 3.5-1: Profile in garnet on either side of a fracture containing orthopyroxene and plagioclase. |
Fig. 3.5-2: Times needed to produce a profile of 15 µm penetration depth for a given temperature and for the different diffusion data sets shown. |
There is a possible flaw with this type of modelling. What if grain boundary diffusion is really not as fast as many have suspected - especially in rocks poor in a free fluid phase and in which notoriously slow diffusers such as plagioclase and pyroxene are major phases? Perhaps the result is that the migration of material to and from the garnet boundary is as slow as or even slower than the internal diffusion (consider the larger transport distance compared to the profile penetration depth). In this case the assumption of a fixed rim composition breaks down, the model is inappropriate, and the result is invalid. Does this mean that the implied fast cooling rates are also false? In order to overcome the grain boundary diffusion problem let us consider some more spatially constrained compositional information.
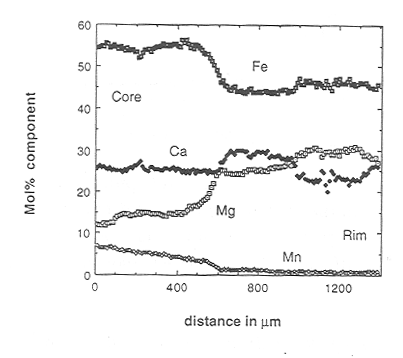
The larger garnets in the sample show a distinct red coloration in their
cores that contrasts with pale pink to colourless rim zones. The two-dimensional
element distribution in garnet revealed by X-ray mapping shows astonishingly
sharp compositional changes that allow the garnets to be subdivided into
a core region and two overgrowth zones. The boundaries between the zones
are irregular - no idiomorphic faces are present - and the outer zone is
incomplete. Conventional line profiles show that there are sharp steps
in composition between the different zones (Fig. 3.5-3). If the boundary
between the core and the first overgrowth is presumed to have been initially
a sharp step in the profile, then the time taken to develop the diffusion
profile between these two segments of garnet can determined by an interdiffusion
model (taking into consideration the volume differences of course). This
problem is currently being tackled. The preservation of such sharp compositional
features in rocks that have experienced high temperatures can only be explained
if exposure to high temperatures was only for a short time and this puts
serious constraints on tectonic models. Even more spectacular in this sample
is the zoning in much smaller, sub-mm, garnets (Fig. 3.5-4), where the
volume effect for diffusion should be more marked but in fact the zoning
is equally as sharp. This is an unequivocal natural test of the diffusion
data that makes it difficult to argue that the experimental diffusivities
are too slow: faster diffusivities would require even more spectacular
heating and cooling rates in nature!
 |
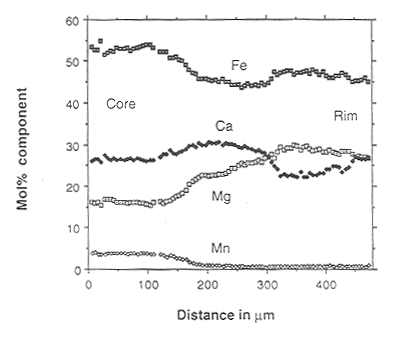 |
Fig. 3.5-3: Core-rim profile in garnet showing the three compositional zones. |
Fig. 3.5-4: Core-rim zoning in a small garnet from the same sample also showing three zones. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page