

It is possible to determine directly the activities of the oxide components
MgO, Al2O3, or SiO2 in melts and minerals
through analysis of Pd alloys equilibrated with them at fixed T and fO2.
The activity of each component relative to the pure solid oxide is a simple
function of the ratio of the Mg, Al, or Si dissolved in Pd equilibrated
with the sample to the Mg, Al, or Si in Pd equilibrated with the corresponding
oxide. Previous experimentation suggested a dependence of the activity
coefficients of MgO, Al2O3, and SiO2 on
composition for simple silicate liquids in the system CaO-MgO-Al2O3-SiO2-TiO2
(CMAST) that were synthetic analogs of Ca,Al-rich inclusions in carbonaceous
chondrites. Measurement of oxide activities have now been performed on
compositions in the "basalt tetrahedron" (forsterite-diopside-CaTschermaks-silica)
in the system CaO-MgO-Al2O3-SiO2 (CMAS),
with the intent of extending the study to more silicic compositions and
lower Al:Si ratios, and providing a simple model system in which changes
in thermodynamic properties as a function of differentiation in an Fe-free
basaltic system may be observed. Comparison between activities determined
experimentally at 1250° - 1400 °C and those predicted by published
models shows good agreement overall, although the SiO2 activities
are systematically high. Plots were made of the activity coefficients γi
of the various oxide components as a function of composition using both
the prior data on the meteoritic liquids and the new data on the Fe-free
basaltic liquids. A strong positive correlation exists between γSiO2
and XSiO2, which probably indicates the controlling influence
of SiO2 as a network former (Fig. 3.7-7). There is also a good
negative correlation between γAl2O3
and the number of non-bridging oxygens per total oxygens in the melt (NBO/Σ
O), implying that
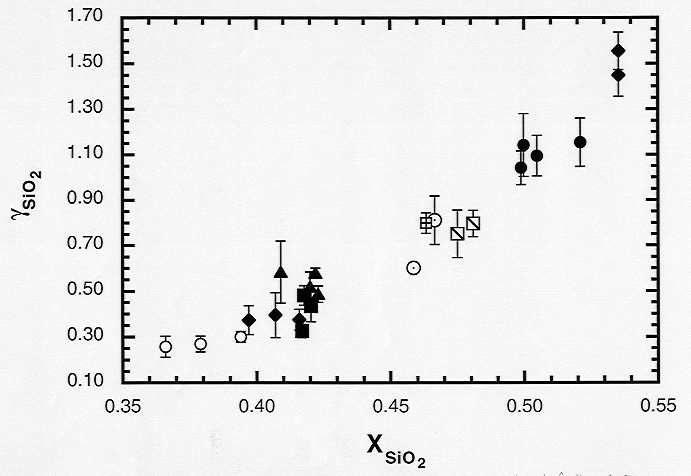 |
Fig. 3.7-7: Activity coefficient of SiO2 in silicate melt as a function of mole fraction of SiO2. |
depolymerization of the melt results directly in the breakup of Al - O bonds. However, γSiO2 increases either with decreasing NBO/Σ O or decreasing Al:Si ratio, such that there is no correspondingly simple relationship between γSiO2 and NBO/Σ O. This implies that network modifiers preferentially attack Al - O bonds as they break up the silicate framework. The activity coefficient of MgO exhibits a well-defined negative correlation with XSiO2 and a positive correlation with XCaO, implying an overall negative relationship between gMgO and the degree of polymerization. However, there is no clear correlation between γMgO and NBO/Σ O, perhaps due to the presence of the second network modifier CaO. The effects of differentiation in these liquids may be observed in the increase of γSiO2 as the silica-poor phases forsterite and pigeonite crystallize from the melt.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page