

It is commonly assumed that if an aluminosilicate melt contains metal cations (M, e.g. Na+, Ca2+) in excess of those required to charge balance aluminium, then the latter are stabilized entirely as network formers in tetrahedral coordination. If insufficient cations are available, spectroscopic data suggest that aluminium occurs as a network modifier with an increased coordination number. Therefore, at fixed silica content, if aluminium is substituted for metal cations, a change in structural role should occur at the metaluminous join (M/Al=1). Because melt viscosity is a very sensitive function of melt composition (and in some way melt structure), such a change in the structural role of aluminium should be manifest in melt viscosities at, or close to the metaluminous join.
The shear viscosities of 17 melts in the system Na2O-Al2O3-SiO2
have been determined using the concentric cylinder method. To date, three
series of compositions have been studied, containing between 50 and 75
mol% SiO2, and mol (Na/(Na + Al)) in the range 0.55 to 0.40.
The peak in viscosity at fixed silica content is not found to occur exactly
at the metaluminous join (Na/Al = 1) for any of the studied isopleths of
silica content, but always within the peraluminous field, as shown in Fig.
3.8-3. The viscosity maximum is very close to the metaluminous join for
melts containing 50mol% SiO2, but moves further into the peraluminous
field with increasing silica content occurring close (Na/(Na + Al)) of
0.47 at 75 mol% SiO2.
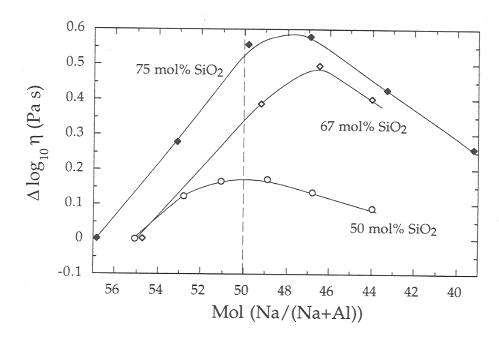 |
Fig. 3.8-3: The variation of melt viscosity at 1550 °C as a function of sodium/aluminium ratio at fixed silica content. The sample preparation and experimental set-up were designed to maximize the precision of the measurements, in order to constrain the location of the viscosity maximum. |
The presence of a viscosity maximum in the peraluminous field is difficult to reconcile with the notion that all aluminium is charge balanced by sodium along the metaluminous join. The simplest interpretation is that some aluminium is not associated with sodium; this proportion getting larger with increasing silica content over the studied range of SiO2 contents. This aluminium could be present as triclusters in octahedral coordination, as has been suggested for peraluminous melts, or alternatively may occupy a charge balancing role for tetrahedral aluminium. Because melt structure controls not only the physical properties of silicate melts, but also the thermodynamic properties, the present data raise the possibility that many geologically important components (e.g. water) may have minimum values of solubility, not at the metaluminous join, but within the peraluminous field. Given that many granites, diorites, and tonalites lie close to the metaluminous join in composition, and have silica contents comparable to those studied here, these results may have important implications for the physical and chemical evolution of such natural systems.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page