

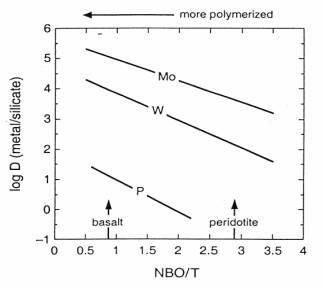
To explain siderophile element overabundance in the Earth's upper mantle, different models of Earth accretion and core segregation have been proposed. To constrain these models, it is important to have data on the partitioning behaviour of these elements as a function of oxygen fugacity, temperature, pressure and melt composition. Melt composition in particular is a variable that might be important in siderophile element partitioning behaviour. Although it appears to play a minor role in Ni partitioning, in comparison to temperature and oxygen fugacity (Annual Report 1998), it has been observed that the siderophile character of Mo and W are affected by melt composition. Both elements become less siderophile as the melt becomes more depolymerised (Fig. 3.3-6).
Although melt composition seems to play a major role in the partitioning of W and Mo in silicate melts, there are few experimental studies on this topic. The present work reports new data concerning the effect of sodium metasilicate on Mo and W solubilities in silicate melt (anorthite-diopside eutectic composition). Two individual experiments were performed at 1400°C under reducing conditions using the stirred crucible technique developed by Dingwell et al. (1994). Major elements, and W and Mo contents in the glass samples were determined using the electron microprobe.
Figure. 3.3-7 illustrates the variation of tungsten and molybdenum solubility, normalised to the solubility in pure anorthite-diopside eutectic composition melt, as a function of the sodium
 |
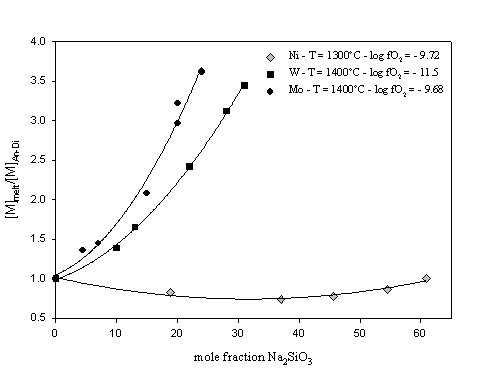 |
metasilicate content of the melt. For comparison, data from a similar study on Ni performed by Ertel et al. (1997) are also reported.
Tungsten and molybdenum solubilities increase with addition of sodium metasilicate, and the observed effect is significantly larger than for Ni solubility. This increase in W and Mo solubility might be explained by structural melt properties and the formation of very stable metal-alkali complexes in the melt. In comparison to Ni, W and Mo cations may have a greater affinity for sodium cations.
The present results, which are in very good agreement with several previous studies, imply that the more basaltic (i.e. depolymerised) a melt is, the greater is the partitioning of W and Mo into the melt. This compositional effect may be much stronger for elements such as W and Mo which have high oxidation states in silicate melts (valence of +4 for W and +4 and +6 for Mo), than for lower valence elements like Ni and probably Co (+2 valence in silicate melt).

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page