

The development of double stage cubic-anvil type apparatus enables the behavior of materials to be studied precisely under conditions corresponding to the uppermost part of the lower mantle. An increase in the pressure range requires the use of other techniques. Although diamond anvil cells are used widely at higher pressures, it is very difficult to uniformly heat the sample and to keep these conditions stable. A "Drickamer-type" opposed anvil apparatus modified for high-temperature experiments was successfully used to study the behavior of MgSiO3 perovskite under deep lower mantle conditions but this system was very difficult to use and no further studies have been successfully made since then. In order to develop a technique for routine use, improvements were made with regard to the pressure transmitting materials, sample assembly, and other areas of the experimental techniques.
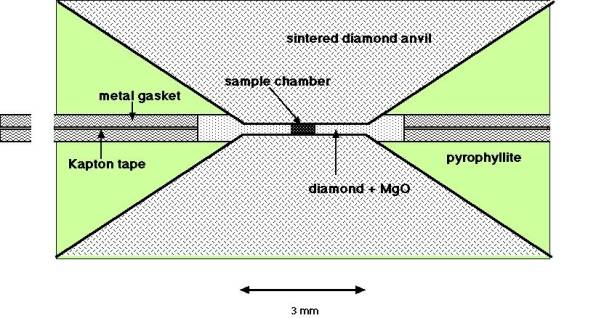
Anvils were made of sintered diamond (15 mm in diameter and 15 mm in height) with a 3 mm diameter culet. Fig. 3.7-3 shows a typical sample assembly used in the present study. The use of a metal gasket and a mixture of diamond powder and MgO, instead of a mixture of boron and epoxy resin, in the previous study, enabled the generation of high, stable pressures. As an example for a pressure calibration we have used GaP, which shows a very sharp resistance drop of seven orders of magnitude when applying only 7.5 tons to the anvils. The sharpness of the resistance drop indicated that, contrary to tungsten carbide anvils, the pressure generating efficiency was not reduced in this pressure range (at about 23 GPa). Heating under high pressure requires the development of a furnace which fits into this sample chamber. The cobalt metal binder enables the sintered diamond anvils to be used as part of the heating circuit. Metal foil electrodes then connect the internal ceramic furnace with the sintered diamond culets. As the sintered diamond has a relatively high resistance special care was required to reduce the contact resistance at the surface of the anvil otherwise heating occurs in this region and the foil electrodes melt. It was also desirable to use heater materials which have high resistance. We have tried LaCrO3 and a mixture of diamond powder + TiC. Commercially available LaCrO3 has grain sizes that are too large in order to machine this material for the small assembly. However use of the mixture of TiC + diamond powder is promising. Another future task is to establish a way to put a thermocouple into the small sample chamber.
The size of the sample chamber of this apparatus is much smaller than that of the multi-anvil apparatus. However, compared to the diamond anvil cell, the sample volume is about one order of magnitude larger and there are many options to achieve stable, uniform sample temperatures. The use of recently developed micro-fabrication techniques will be indispensable for the further development of this apparatus.
 |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page