

The solubility of noble metals (Pt, Rh, Ir) in silicate melts has been investigated using the mechanically assisted equilibration technique. The experiments, performed under carefully controlled sequences of oxygen fugacity and temperature conditions provide data useful in modelling the partitioning of siderophile elements during the early evolution of the Earth. During the last year experiments have been performed to address the question of metal solubilities especially at very low oxygen fugacities.
An Ir solubility experiment was performed with forming gas (N2/H2
mixture) over an ice-water mixture instead of CO/CO2 gas mixtures.
With this gas mixture the oxygen fugacity was fixed at 1340 °C at log
fO2 = - 11.8. In addition, to expand the range of investigated
oxygen fugacities to higher values we applied pure CO2 and oxygen-argon
mixtures of increasing oxygen contents (1 %, 3 %, 10 %, as well as pure
air) to the Ir solubility experiment. Figure 3.2-2 shows the latest results
from our Ir experiments compared to results of our previous Ir experiment
at 1400 °C. The difference in absolute Ir solubilities observed between
the present and the previous experiment is due to a temperature difference
(1400 °C vs. 1340 °C).
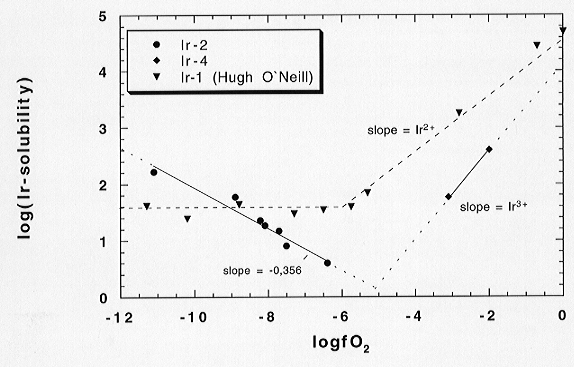 |
Fig. 3.2-2: Comparison of Ir solubility experiments. Ir solubilities (in log units) are plotted against logfO2 of the applied gas mixtures. Black dots and triangles are from Ir experiments applying CO/CO2 gas mixtures; black squares refer to CO2 and Ar/oxygen mixtures. |
At fairly high oxygen fugacities (above log fO2 = -5) the solubility of Ir in the melt increases with increasing oxygen fugacity for both experimental approaches. This is consistent with a valence state of Ir in the melt as Ir3+ (or less). In the fO2 regime controlled by CO/CO2 gas mixtures (below log fO2 = -6) the solubility of Ir stayed constant in the previous experiment, presumably due to a lack of equilibrium during that experiment. In our present Ir experiments (of longer duration) the solubility of Ir starts to increase again with further decreasing oxygen fugacity, correlating well with the increase of p(CO) in the gas mixtures. One interpretation of this observation is the formation of Ir carbonyls or other Ir-C-interactions in the melt. To evaluate this hypothesis, the experiment was immediately reversed to higher oxygen fugacities again resulting in decreasing contents of Ir in the melt as expected. Results from ICP-MS measurements performed at ANU (Canberra) show evidence for a decreased solubility under very reducing conditions which supports the carbonyl hypothesis. INAA (Mainz) data, however, show increased Ir solubilities in corresponding samples. A final check on the carbonyl hypothesis awaits a resolution of analytical discrepancies.
Pt and Rh solubility experiments have not been possible in the past
because of the lack of an appropriate and reliable analytical technique.
ICP-MS measurements (Canberra, ANU) have now made analysis possible. First
measurements demonstrate an excellent accuracy and extraordinary low detection
limit of this technique. As a result we have begun to perform solubility
experiments with a Pt/Rh alloy crucible and spindle. Beginning in air the
oxygen fugacity was slowly decreased by applying CO/CO2 gas
mixtures. The results from the ICP-MS measurements to date are shown in
Fig. 3.2-3. Pt and Rh seem to show the same
 |
Fig. 3.2-3: ICP-MS results from Pt and Rh solubility experiments. Solubilities (in log units) are plotted against oxygen fugacity (in log units). Indicated slopes of the regression lines for the real oxidation state of the element (Pt1+ and Rh2+) in the melt are supplied. |
solubility behaviour in respect to the application of CO/CO2 gas mixtures at rather low oxygen fugacities as for Ir: below an oxygen fugacity of about log fO2 = - 5.5, a further decrease of fO2 leads to increasing Pt and Rh solubilities (carbonyl- formation?).
Based on the present results the investigated platinum group elements (e.g., Ir, Pt, Rh) seem to dissolve in the melt either in the form of carbonyl species or in consequence of a carbon interaction at very low oxygen fugacities when controlled by CO/CO2 gas mixtures. The chemical potential of carbon in basaltic melts at typical terrestrial fO2's may therefore be sufficient to control the geochemical behaviour of Ir, Pt and Rh.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page