

Columbite solubility in granitic melts has been determined as a function of melt composition, temperature (800 - 1035 °C), pressure (0.8 - 5.0 kbar) and melt water content (0-7 wt% H2O). Solubility increases with alkali content in peralkaline melts such that relative to the sub-aluminous composition (mol Al = Na + K), one additional mole of Ta or Nb is added to the melt with each two additional moles of Na+K (Fig. 3.7-8). This suggests complexing of Ta and Nb with 2 non-bridging oxygens. The solubility products of the Ta- and Nb-endmembers are similar for peralkaline compositions but the solubility product of MnTa2O6 is three times lower than that of MnNb2O6 for peraluminous compositions, which may explain Ta/Nb fractionation in the Earth's crust. Solubility strongly increases with temperature and water content in subaluminous to peraluminous melts, in contrast to peralkaline melts which have only a weak dependence. Pressure does not strongly influence solubility but there is an apparent opposite effect; with increasing pressure solubility increases in subaluminous melts and decreases in peralkaline melts.
SnO2 solubility was determined at different fO2
conditions by the diffusion profile method for a suite of water-saturated
peralkaline to peraluminous granitic melts at 850 °C and 2 kbar. Solubility
increases strongly with alkali content in peralkaline melts and also weakly
with Al content in peraluminous melts. For sub- to peraluminous compositions
a sharp break in slope in log solubility versus log fO2
space defines the Sn4+ to Sn2+ transition. This transition
becomes progressively weaker with increasing alkali content in peralkaline
melts, reflecting a progressively higher Sn4+/Sn2+
ratio at fixed fO2. Diffusivity also varies with fO2
and the effective binary diffusion coefficient for Sn2+ is 10-7.5
cm2/sec whereas for Sn4+ it is 10-8.2
cm2/sec.
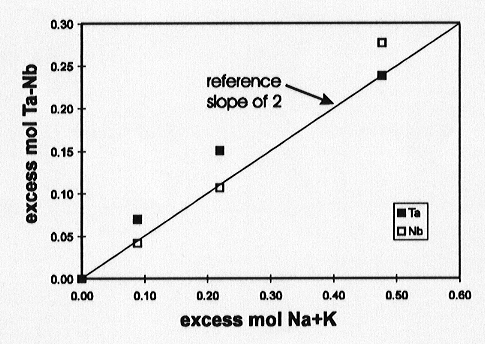 |
Fig. 3.7-8: Solubility of MnTa2O6 and MnNb2O6 in water-saturated peralkaline melts at 800 °C and 2 kbars relative to the solubility in a water-saturated subaluminous (Al = Na + K) melt. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page