

There have been numerous attempts to relate chemical diffusivities in
silicate melts to melt viscosity. For example, the Stokes-Einstein equation
states that Dη= kT/6¶r
where D is the chemical diffusivity, η is the
melt viscosity, k is Boltzmann's constant, and r is the radius of the diffusing
species. Simple relations such as the Stokes-Einstein equation fail to
predict diffusivities for most elements; however our recent work on diffusivities
of U and Th revealed that the activation energy of diffusion was in some
cases equal to that of viscous flow. We have performed several tracer diffusion
experiments on a haplogranitic base composition whose viscosity is controlled
by the addition of Na2O and H2O. The base composition
is HPG8 (79.1 % SiO2, 12.1 % Al2O3, 4.6
% Na2O, 4.2 % K2O), a haplogranite near the minimum
melt composition whose properties have already been exhaustively studied
in Bayreuth. The peralkaline equivalent, HPG8+20, has had 20 wt% Na2O
added to it; hydrous compositions contain 2 or 5 wt% added H2O.
Diffusion profiles are being measured by ion microprobe at Nancy and by
electron microprobe at BGI. Diffusivities of all 22 elements studied (Li,
Be, B, Mg, Ca, Ti, Ge, Rb, Sr, Y, Zr, Nb, Cs, Ba, Nd, Tb, Lu, Hf, Ta, W,
U, Th; 1000 ppm concentration of each element) are Arrhenian; activation
energies of diffusion of all elements in each melt composition are similar,
and are close to activation energies of viscous flow. However there are
large systematic variations in the relative magnitudes of the diffusivities
within groups of the periodic table (Figure 3.8-2). An increase in ionic
radius is correlated with increasing diffusivity within groups IIa and
IIIb (including lanthanides), but is inversely correlated with increasing
diffusivity in group IVb. The results indicate that diffusivities of these
elements can be predicted within an order of magnitude from melt viscosity
over a wide range of conditions and compositions.
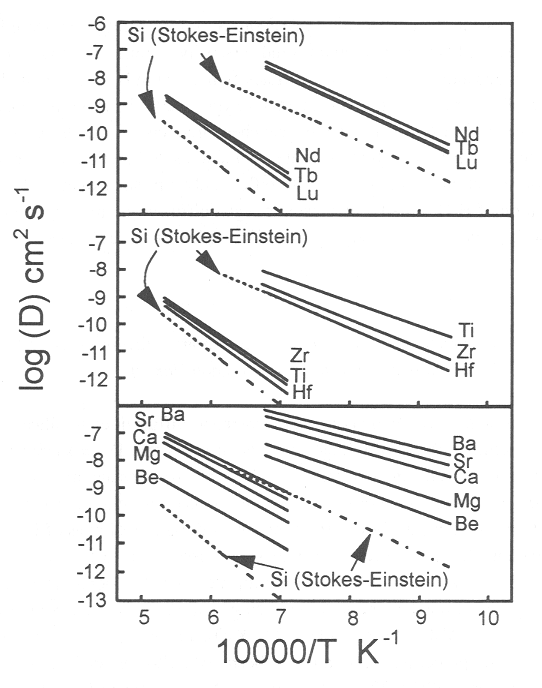 |
Fig. 3.8-2: Log diffusivity versus reciprocal temperature. Diffusivities shown for Be, Mg, Ca, Sr, Ba, Nd, Tb, Lu, Ti, Zr, Hf in HPG8 and HPG8+20 Na2O melts. Shown for comparison are the diffusivities of Si predicted from the Stokes-Einstein relation based on melt viscosities measured by concentric cylinder viscometry. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page