

Based on limited spectroscopic investigations (NMR, Raman, IR and XANES) it is clear that the solution behaviour of phosphorus in aluminosilicate melts is strongly dependent on composition, in particular, the alkali/aluminium ratio. A tentative model of the structural role of phosphorus has been suggested which describes the formation of alkali phosphate complexes in peralkaline melts (x = Na/Na + Al > 0.5), the polymerisation of these complexes to more extended chains in metaluminous (x = 0.5) and peraluminous (x < 0.5) melts and the formation of AlPO4 complexes in the latter systems. In consequence, the addition of P2O5 should lead first to a higher polymerisation and at a crucial ratio x to a depolymerisation of the aluminosilicate network.
Previously published spectroscopic investigations do not provide unambiguous
results, and a comprehensive picture of the solution mechanism of phosphorus
in aluminosilicate melts. Therefore, in conjunction with physical property
measurements (see 3.8d), 31P MAS NMR investigations on the system
xNa2O:(1-x)Al2O3:2Si2O containing
up to 7 mol% P2O5 were performed to monitor the formation
of various P-bearing species. The compositions studied ranged from x=1
to x=0.44; the respective 31P MAS NMR spectra are shown in Fig.
3.8-5. In the spectra of the peralkaline samples with x=1....0.7 the resonances
of Na3PO4 monomers (15 ppm) and Na4P2O7
dimers (2 ppm) can be identified. The relative intensities of these peaks
is a function of the P2O5 content as proposed by
the above mentioned model. Furthermore, the relative intensity of two signals
(shoulders at 7 ppm and -7 ppm) increases as x decreases; these two resonances
are tentatively assigned to end- and middle groups of more extensive chains
(NaPO3)n. The spectra of the peralkaline samples
show clearly that the Na-P complexes become more polymerised with increasing
P2O5 content and decreasing peralkalinity. The spectra
of met- and peraluminous samples contain only one broad, almost featureless
peak which does not vary significantly as a function of phosphorus content.
The chemical shifts increase as a function of the aluminium content and
approach the range typical of AlPO4 species for the samples
with a peraluminous composition. Conclusions drawn from the 31P
MAS NMR investigations agree well with those inferred from macroscopic
property measurements, but a number of questions still need to be investigated
in detail. Further work will concentrate on an unambiguous peak assignment
including two-dimensional NMR experiments, quantitative determinations
(e.g. calculation of average (NaPO3)n chain lengths),
the investigation of the influence of experimental conditions such as quench
rate and traces of water within the melts, and the observation of the network-formers
silicon and aluminium.
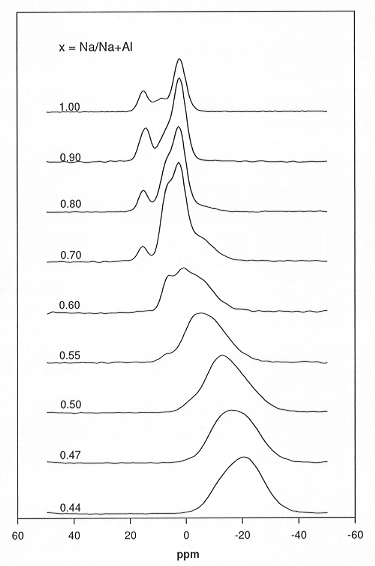 |
Fig. 3.8-5: 31P MAS NMR spectra of xNa2O:(1-x)Al2O3:2Si2O glasses containing 2.5 mol% P2O5. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page