

Bubble growth during the ascent of magmas changes the physical and chemical properties of the silicate
melt, the flow properties within the magma conduit and therefore ascent
rates. All of these processes are important for defining the eruption regime.
We have investigated the nucleation and growth of water bubbles in silicic
melts using a hydrothermal externally-heated diamond-anvil cell (DAC).
This device allows experiments to be performed at magmatic pressures (4
to 25 kbars) and temperatures (T < 950°C) with direct observation
of the processes occurring inside the sample chamber. The experimental
procedure consists of filling the sample chamber of the DAC with a chip
of haplogranite glass (78.6 wt% SiO2, 12.5 wt% Al2O3,
4.6 wt% Na2O and 4.2 wt% K2O), water and an air bubble,
and heating the chamber to increase pressure. This causes the melt to hydrate
at high temperature and pressure. Temperature is measured directly during
the experiment, while pressure is calculated afterwards using the equation
of state of water (assuming isochoric behaviour in the sample chamber).
Following melt hydration, switching off the power to the heater causes
the sample to cool, at a rate of ~32°C/s, which results in a pressure
decrease P. During pressure decrease,
water exsolves from the melt and bubbles nucleate and grow until the glass
transition is reached. The entire experiment is observed through an optical
microscope and recorded by video so that the bubble nucleation, bubble
growth, and glass transition are monitored directly. Bubble sizes are measured
on frame-captured images every 0.2 s.
P. During pressure decrease,
water exsolves from the melt and bubbles nucleate and grow until the glass
transition is reached. The entire experiment is observed through an optical
microscope and recorded by video so that the bubble nucleation, bubble
growth, and glass transition are monitored directly. Bubble sizes are measured
on frame-captured images every 0.2 s.
Bubbles nucleate and expand in melt globules of sizes between ~ 20 to 70 µm in radius (Fig. 3.4-8), but nucleation does not occur in melt globules with radii < 15 µm. Comparing the timescales of bubble nucleation (directly observed) and water diffusion (calculated)
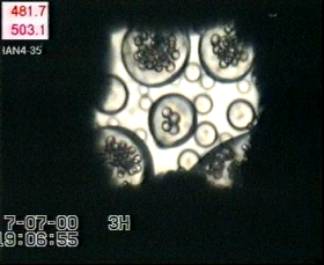 |
suggests that in a 15 µm-radius melt globule, water diffuses into the surrounding water medium before bubbles
have time to grow. In melt globules with radii > 15 µm, bubbles reach
3.6 to 9.1 µm in radius within 6.1 to 11.7 s (until the glass transition
is attained) while the temperature decreases by 192-313°C, corresponding
to  P of 2.9-6.6 kbars (
P of 2.9-6.6 kbars ( P
rate of ~0.5 kbar/s).
P
rate of ~0.5 kbar/s).
Bubble nucleation iseither a single event occurring within the first second or successive pulses over a period of up to 7 s. The successive nucleation events occur when the melt globules are in contact with the lower-diamond culet of the cell, the interface providing a preferential nucleation site for water (bubbles appear with a typically flattened shape). This localised early nucleation probably induces heterogeneities in the melt water content that lead further to successive nucleation pulses. The various bubble sizes generated by successive nucleation events may explain the polymodal bubble size distributions commonly observed in natural magmas (especially where bubble coalescence can be dismissed as an explanation).
Bubble growth (radius R as a function of time t) does not follow the "classical" square-root of time law for the isothermal case, but rather follows a logarithmic law (R=A*Ln(t)+B, where A and B are constants determined for each run), as shown in Fig. 3.4-9. We explain the deviation from a square-root of time to a logarithmic law by the temperature drop during the
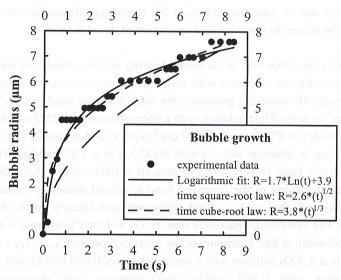 |
pressure decrease that significantly affects major parameters involved in bubble growth, such as viscosity, diffusivity, melt and gas densities. Comparing the observed glass transition temperature with those calculated at P < 2.5 kbars (recalculated for an average cooling rate of 32°C/s) suggests that pressure has little influence on the glass transition temperature in the range 3.4 to 15.2 kbars.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page