

It is widely accepted that a large part of the Earth was molten during its early history, thus forming a `magma ocean'. Silicate melt - (liquid) metal interaction during the separation of core material, as well as mineral-melt interaction during the cooling of the magma ocean, are therefore among the possible reactions that took place in the accreting Earth. The extent to which these different reactions have contributed to establishing current mantle abundances of siderophile elements is the object of ongoing investigations and dispute. The importance of silicate melt composition ( Xmelt) for the distribution of siderophile elements between liquid metal and silicate melt has recently been explored, thus introducing an additional parameter to P, T and fO2 that has to be considered when interpreting the results of partitioning studies. Large inconsistencies between existing studies (e.g. McFarlane et al, GCA 58, 23, 5161 (1994); Agee, Nature, 346, 834 (1990)) on magnesiowüstite-silicate melt distribution coefficients (DMw/Sil) may possibly be resolved by considering differences in melt composition. Moreover such experimental data may provide new insights into the question of whether mineral fractionation during the early history of the Earth contributed to differentiation processes in the mantle.
We performed element partitioning experiments between magnesiowüstite and silicate melt using a multi-anvil apparatus at 9 to 25 GPa and 1800° to 2500°C for 2 to 20 min. Samples were analysed using a CAMECA SX50 electron microprobe. The two element partitioning coefficients KDMw/Sil between magnesiowüstite and silicate melt are defined as KD(M/Mg) Mw/Sil = (cMMw/cMSil ) / (cMgMw /cMgSil ). Instead of the usually employed structural parameter NBO/T, we use other chemical parameters such as the Si/Mg ratio in the quenched silicate melts, which serve equally well for investigating the partitioning behaviour as a function of melt composition.
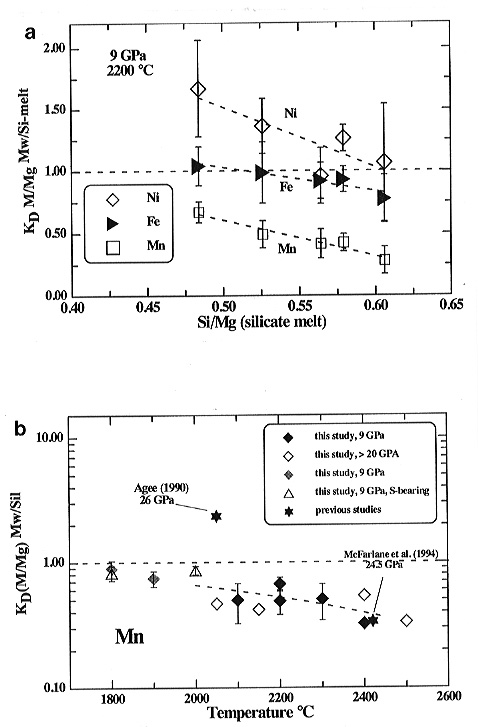
The KDMw/Sil obtained for Fe, Ni, Co, Mn, Cr and
V exhibit dependencies on both temperature (at constant Xmelt)
and melt composition (at constant T) at 9 GPa. As an example, the partitioning
of some elements between magnesiowüstite and silicate melt as a function
of melt composition and temperature at constant pressure are shown in Fig.
3.2-1a and 3.2-1b. Additional experiments at pressures above 20
GPa exhibit distinct pressure effects for the partitioning behaviour of
some elements (e.g. Co, Cr, V) while almost identical KDMw/Sil
values were determined for other elements (e.g. Mn, Fe) at high and
low pressures (e.g. Fig. 3.2-1b). In the present experiments Mn
preferentially partitions into silicate melt while Ni and Co are more compatible
in magnesiowüstite irrespective of the P, T, or melt composition of
the experiments. Cr and V prefer silicate melt at high pressures and magnesiowüstite
at low pressures. Our results generally agree with those of McFarlane et
al. (1994) and the pressure, temperature and composition effects on magnesiowüstite-silicate
melt partitioning determined here cannot solve the inconsistencies (i.e.
significantly different KDMw/Sil at comparable P,
T conditions) between McFarlane et al. (1994) and Agee (1990). In contrast
to the other experiments reported here S-rich metal liquid was present
in the experiment of Agee (1990). However, additional S-bearing experiments
in our study clearly show that sulphur has a negligible influence on oxide-silicate
partitioning (Fig. 3.2-1b). Because all experiments have been performed
at oxygen fugacities at or below the iron-wüstite buffer, the presence
of ferric iron in magnesiowüstite and/or silicate liquid is unlikely
to be responsible for the differences between the various studies. Our
study indicates that other variables, for instance compositional effects
such as the magnesiowüstite composition (e.g. its FeO content) may
be responsible for the observed differences between literature data. Further
experiments are underway to investigate this possibility.
 |
|
(b) Magnesiowüstite-silicate melt partitioning coefficients for Mn as a function of temperature at 9 GPa and 20 to 25 GPa in comparison to literature data. The regression line is given for the 9 GPa results. The triangles denote S-bearing experiments. Grey symbols (experiments below 2000°C) show partitioning between solid silicate and magnesiowüstite. |
The present experimental results contribute important information to the discussion about the extent to which mineral fractionation processes were responsible for the observed siderophile element anomaly in the Earth´s mantle. In particular the abundances of Mn, Cr and V are difficult to explain by simple metal-silicate equilibrium processes. Because none of the determined KD values for these elements significantly exceeds unity, the present results indicate, in accordance with McFarlane et al. (1994), that it is unlikely that magnesiowüstite fractionation has contributed significantly to the abundance patterns of the investigated elements in the mantle.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page