

The origin of carbonatites remains a contentious topic. However, an important role for liquid immiscibility between silicate and carbonate liquids has often been proposed. To understand and constrain the role of this process in carbonatite generation, it is important to have trace element partitioning data. Few experimental studies on trace element partitioning between silicate and carbonate liquids have been undertaken, reflecting both analytical and experimental difficulties. For example, the carbonate liquid does not quench well, and concentrations of trace elements are often difficult to determine in-situ. It is not possible to avoid contamination when hand-picking the phases, or acid leaching of run products. To circumvent some of the problems, new two-liquid experiments have been made utilizing the rotating centrifuge autoclave, and the trace elements in the run products analysed using the laser ablation microprobe.
A glass corresponding in composition to the NaAlSiO4 - Na2Si2O5 eutectic in the SiO2 - Al2O3 - Na2O system and reagent-grade Na2CO3, K2CO3, MgCO3 and Ca3(PO4)2 are major components of two synthetic reactant mixtures used in the runs. The first mixture was doped with selected rare earth elements (La, Nd, Sm, Tb, Er, Tm), Sr, Ba and Y; the second one was spiked with a number of high field strength elements (Zr, Hf, Nb, Ta and Ti). Trace elements were added as pure oxides and mixed by grinding. About 80 mg of the mixtures were sealed in Pt capsules and preheated for 24 hours in a rapid-quench cold-seal pressure vessel to achieve the immiscibility and equilibration of the conjugate liquids at P-T conditions identical (or very close) to those in subsequent centrifuge runs. The capsules were quenched and placed, for one hour, in the rotating autoclave for high-temperature centrifuge separation. The separation was performed at an acceleration of 500 g, at temperatures 965-1015oC and pressures 840-920 bar. These conditions were sufficient for the liquids to separate and form two distinct immiscible layers separated by a sharp meniscus. The less dense carbonate layer formed a fine-grained crystal aggregate on quenching. The samples easily split along the meniscus without contamination.
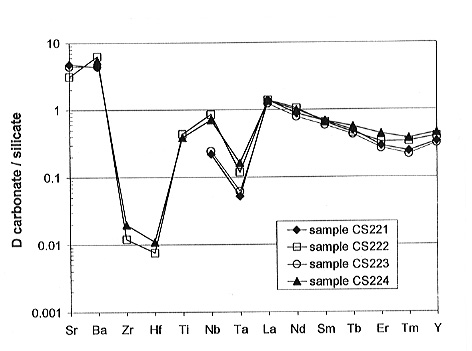
The partition coefficients for trace elements are illustrated in Fig. 3.2-2. Sr and Ba are the only elements that show a strong preference for the carbonate liquid. Ti, Nb and the REE (excluding La) are partitioned preferentially into the silicate liquid, with D values lying in the range from ~0.2 to 0.9. Zr, Hf and some Ta results indicate D values less than 0.1, with Zr and Hf having values of about 0.01. The effect of these variations in D is to fractionate Sr and Ba strongly from the HFSE and REE, and to cause a strong fractionation within the HFSE. It should be also noted that fractionation between the immiscible liquids results in decoupling of so-called "geochemical twins" like Zr and Hf, or Nb and Ta.
 |
|
|
It is noteworthy, however, that the data on partition coefficients are in drastic contrast to the broad occurrence of Nb (pyrochlore) and Zr (baddeleyite) ore deposits in carbonatites. Low carbonate/silicate partition coefficients for HFSE suggest a residual nature for the ore-bearing carbonatites, rather than their origin by liquid immiscibility.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page