

During recent years, a growing number of studies have focused on the solubility
of water in nominally anhydrous minerals, as well as on the stability field
of dense hydrous magnesium silicates. The results are of major importance
since water can dramatically influence the physical properties of the Earth´s
mantle such as melting, transport properties, and rheology. (Mg,Fe)SiO3-perovskite,
which constitutes up to 80% by volume of the lower mantle, is believed
to be a potential canditate for water storage in the deep Earth. We conducted
experiments on water partitionning in the system MgSiO3 + 0.5wt%
H2O + excess silica at P,T conditions corresponding to the top
of the lower mantle using a multi-anvil press. Water contents were calculated
from FTIR absorption measurements on optically clear crystals using the
calibration curve of Paterson. Below 24 GPa at 1600°C, the ilmenite
polymorph of MgSiO3 coexists with stishovite and a melt. The
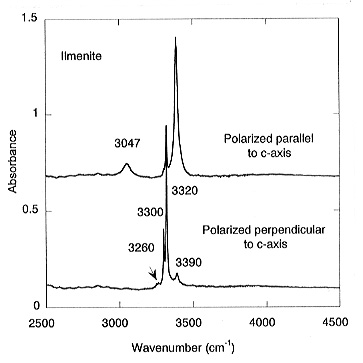
polarized spectra of ilmenite shows five pleochroic bands: three sharp
and strong ones at 3390, 3320 and 3300 cm-1 and two weak bands
at 3260 and 3047 cm-1 (Fig. 3.5-1). The calculated water
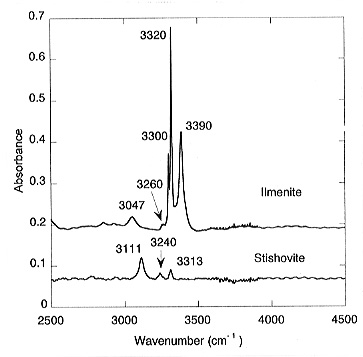
content for ilmenite is 1750 ppm by weight. For stishovite coexisting with
MgSiO3-ilmenite, the measured absorbance yields a water content
of 35 ppm (Fig. 3.5-2).
 |
|
 |
|
|
 |
|
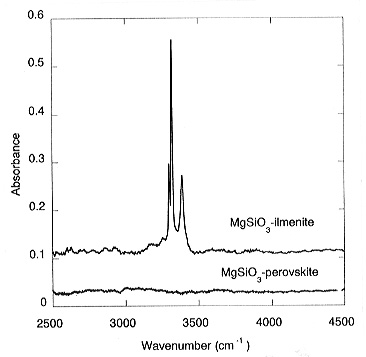
This yields a partition coefficient of water between MgSiO3-ilmenite and stishovite of Dilm/stish = 50. Large amounts of water were also found in the quenched melt; however, the absolute concentrations are difficult to quantify. With increasing pressure, perovskite becomes the stable polymorph of MgSiO3. In a sample where ilmenite and perovskite coexisted, no water was detected in the perovskite while the ilmenite dissolved about 2000 ppm, indicating that Dilm/per >>1 (Fig. 3.5-3).
This observation would imply that MgSiO3 perovskite is not a major host for water in the lower mantle. However, it is conceivable that the solubility of water in this phase could greatly increase in the presence of chemical impurities. Accordingly, solubility experiments are currently being conducted in more complex systems containing aluminium, iron, chromium and other components.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page