

The 1991 eruption of Mount Pinatubo has received considerable attention by Earth scientists due to the enormous amount of sulfur dioxide that was injected into the stratosphere by this volcano. Satellite data suggest that at least 1.7 x 107 tons of SO2 were released. Interestingly, this is much more that one would expect based on the measured sulfur content of the melt prior to and after the eruption, as seen in melt inclusions and in samples of degassed magma. Similar observations during a number of recent volcanic eruptions have led to the suggestion that prior to eruption most of the sulfur was present in a hydrous fluid phase inside the magma chamber. This would imply that hydrous fluids are very efficient in extracting sulfur from a melt; moreover, the distribution of sulfur between fluid and melt at high pressure inside the magma chamber would ultimately determine the sulfur release during eruption. Since SO2 is readily oxidized to sulphate aerosoles in the stratosphere, which are very efficient in blocking solar radiation and inducing global cooling, it would be interesting to study the distribution of sulfur between silicate melts and fluids at the conditions prevailing in magma chambers, i.e. up to several kbar and about 900°C.
Experimental studies involving sulfur at high temperatures and pressures are very difficult because of the need to control oxygen fugacity and because sulfur reacts with most noble metals that are normally used as capsule materials. At temperatures above 700°C, however, gold capsules are sufficiently permeable for hydrogen to allow accurate control of the redox state inside the capsule. Moreover, we were able to show that gold does not absorb measurable amounts of sulfur during high pressure experiments. We therefore used gold capsules in order to study the partitioning of sulfur between melt and fluid over a wide range of temperature, pressure, oxygen fugacity and melt composition. Experiments were carried out in rapid-quench cold seal bombs. Sulfur contents in quenched glasses were measured by electron microprobe with very long counting times; the sulfur content in the fluid phase was calculated by mass balance.
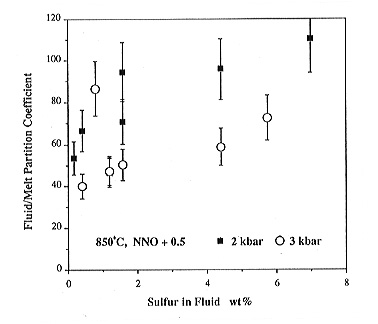
Some selected results on the distribution of sulfur between a haplogranitic
melt and hydrous fluid at log fO2 = NNO
+ 0.5 are shown in Figs. 3.5-11 and 3.5-12. Partition coefficients Dfluid/melt
 |
|
|
are always very high, usually between 20 and 100. This means that even
small amounts of fluid in a magma chamber can extract most of the sulfur
out of the melt, consistent with the sulfur excesses observed during recent
eruptions. In principle, one can use the distribution data in Figs. 3.5-11
and 3.5-12 to calculate the percentage of fluid in the magma chamber required
to yield the amount of excess erupted sulfur. Using data from recent eruptions,
fluid/melt ratios up to 10% are obtained.
 |
|
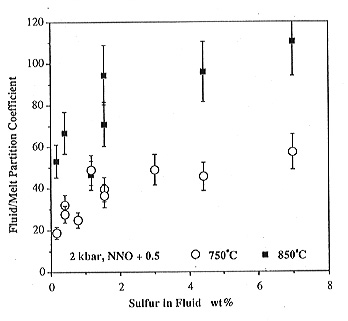
A closer inspection of the data in Figs. 3.5-11 and 3.5-12 reveals some interesting variations in partition coefficients. Dfluid/melt always increases with increasing sulfur content in the system, due to the nonideality of the fluid and melt phase. With increasing temperature and decreasing pressure, sulfur becomes more enriched in the fluid. This explains why increasing sulfur emissions of a volcano often preceed an eruption: if magma rises in the conduit, pressure decreases, which according to Fig. 3.5-11 increases sulfur emissions. Similarily, a batch of new magma entering the magma chamber will increase temperature, which again drives more sulfur into the gas phase (Fig. 3.5-12).

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page