

Knowledge of the structure of silicate melts is of primary importance for understanding magmatic and volcanic processes. X-ray diffraction and Raman spectroscopy studies of silicate melts at magmatic temperatures are very scarce and still represent significant experimental challenges. On the other hand, it is generaly assumed that structural data for glasses quenched from high temperatures can be applied to the melt structure at the glass transition temperature (Tg).
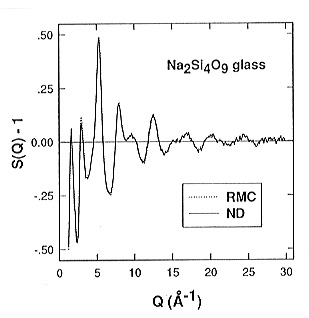
Following this method of investigation, we have studied the structure of the sodium tetrasilicate glass (Na2O.4SiO2, NS4) by neutron diffraction from room temperature up to the glass-liquid transition at about 773 K at the Studsvik neutron research reactor (Sweden). The NS4 glass was selected as a simple model for more complicated geologically relevant glasses and melts. The neutron and X-ray diffraction experiments yield only the so-called pair distribution functions gij(r), which give the probability of finding an atom j at a distance r from an arbitrarily selected atom i at the origin. In order to obtain a three-dimensional model of the NS4 structure at room temperature we have applied the reverse Monte Carlo (RMC) method using high-resolution neutron diffraction data measured previously at the ISIS neutron spallation source (UK).
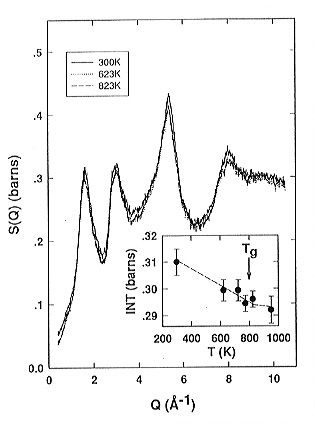
Below the glass transition temperature Tg= 773 K increasing
temperature leads to a gradual decrease of the intensity of the peaks in
the static structure factors (Fig. 3.6-1) and a slight broadening of the
Si-O and O-O peaks in the corresponding pair correlation functions � Fig.
3.6-2. Above Tg the static structure factors remain constant
up to the onset of crystallization. The structural changes observed can
be attributed to temperature-enhanced dynamic distortions of the SiO4
tetrahedra and suggest that there is little change in the short- and medium-range
order of sodium tetrasilicate glass and melt around the glass-liquid transition.
Therefore, the results obtained from the RMC simulations at ambient temperature
could be extrapolated to the melt structure at Tg.
 |
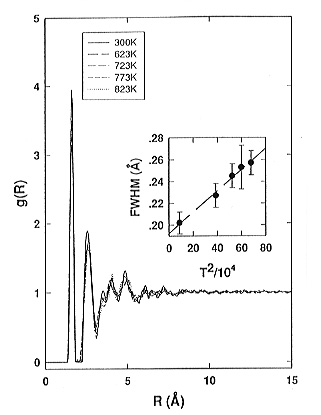 |
| Fig. 3.6-1: Comparison of the total structure factors S(Q) of sodium tetra-silicate glass and supercooled liquid at different temperatures. The inset shows the variation of the height of the peak at about 1.6 Å-1 as a function of temperature. The dashed line in the inset is only a guide for the eye. | Fig. 3.6-2: Comparison of the total pair correlation functions g(r) of sodium tetrasiliate glass and supercooled liquid at different temperatures. The inset shows the dependence of the FWHM of the Si-O1 peak in g(R) at about 1.625Å on the temperature squared. The dashed line in the inset is a linear fit through the data points. The error bars are determined from the least-squares Gaussian fit of the Si-O1 peak. |
Nuclear magnetic resonance results (Q-species distribution) as well
as chemical bonding considerations have been used to constraint the RMC
simulations. Very good agreement with the experimental structure factor
was achieved (Fig. 3.6-3) using 1996 atoms in a cubic box with box edge
30.3Å and periodic boundary conditions. The partial pair distibution
functions gij(r) obtained are consistent with gij(r)
for other sodium silicate glasses determined from molecular dynamics simulations.
The average silicon-oxygen nearest neighbour distance is 1.625 ±
0.011 Å, the average sodium-oxygen nearest-neighbour distance is
2.45 ± 0.40 Å and the average sodium-oxygen coordination number
is 3.6 ± 1.1. The average bond-length distortion of the SiO4
tetrahedra in the RMC model, <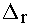 >
= 0.06 Å, compares very well with the static (T = 0 K) bond-length
distortion, <
>
= 0.06 Å, compares very well with the static (T = 0 K) bond-length
distortion, <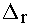 >0
= 0.059 ± 0.002 Å, determined from the temperature dependence
of the full width at half maximum (FWHM) of the first Si-O peak in g(r)
(see Fig. 3.6-2). This result indicates that the thermal broadening of
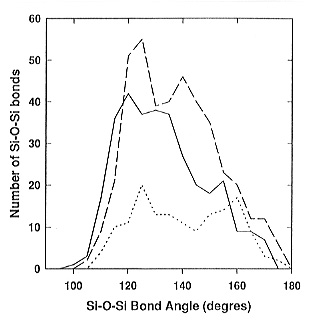
g(r) at room temperature is very small. Analysis of the bond-angle distributions,
short-range order clusters and ring´s statistics revealed that the Si-O-Si
bridging angle distribution is different for the different Qi -Qj
units (Fig. 3.6-4) and a preference for the formation of Q4 -
Q4 units with high strain energy in three-membered rings exists.
This explains why during the initial stages of hydration of NS4 glass,
SiOH groups are preferentially formed by breaking Q4 - Q4
linkages, although a significant number of Q4 - Q3
and Q3 - Q3 linkages also exist in the anhydrous
glass network.
>0
= 0.059 ± 0.002 Å, determined from the temperature dependence
of the full width at half maximum (FWHM) of the first Si-O peak in g(r)
(see Fig. 3.6-2). This result indicates that the thermal broadening of
g(r) at room temperature is very small. Analysis of the bond-angle distributions,
short-range order clusters and ring´s statistics revealed that the Si-O-Si
bridging angle distribution is different for the different Qi -Qj
units (Fig. 3.6-4) and a preference for the formation of Q4 -
Q4 units with high strain energy in three-membered rings exists.
This explains why during the initial stages of hydration of NS4 glass,
SiOH groups are preferentially formed by breaking Q4 - Q4
linkages, although a significant number of Q4 - Q3
and Q3 - Q3 linkages also exist in the anhydrous
glass network.
Calculations are in progress for the vibrational (Raman and IR) spectra
of the NS4 glass based on the generated RMC three-dimensional models, which
would provide more detailed understanding of the character of the vibrational
modes and would serve as test of the usual band assignments of the high-frequency
Raman peaks.
 |
 |
|
|

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page