

The large number of elements present in the cationic sublattice of silicate melts involved in igneous processes stands in stark contrast to the domination of the anionic sublattice by a single element: oxygen. Nevertheless significant deviations from the approximation of a silicate melt as an oxide melt occur in volcanic systems as demonstrated by the high levels of fluorine in topaz rhyolites and chlorine in pantellerites.
The influence of cationic substitutions and mixing on the viscosity of silicate melts has been extensively investigated by the systematic addition of relatively non-volatile oxide components. In contrast, experimental investigations into the influence of anionic substitutions on melt viscosity have been fraught with difficulty. High quality data, most easily obtained at low pressures, are few in number due to the high volatility of many anionic components.
To address this imbalance, shear viscosities of melts in the system Na-Fe-Si-O-F-Cl have been determined over a wide range of temperatures at 1 atm pressure in air. The compositions are based on the addition of Fe2O3, FeCl3 and FeF3 to a base melt composition corresponding to sodium disilicate (Na2Si2O5). Viscosities were determined using concentric cylinder and micropenetration methods and measurements span the range of 100.5 to 1011 Pa s. The chemical compositions of these melts have been analysed after viscometry determinations. The iron is fully oxidized under the conditions of the viscometry. Although fluorine and especially chlorine are volatile elements in silicate melts, levels of chlorine and fluorine up to over 3 and 4 wt%, respectively, have been stabilized in these melts, presumably assisted by the presence of ferric iron. Although some volatilisation occurs during the original synthesis of these samples none occurs during viscometry.
The anionic substitutions Cl2O-1 and F2O-1
have very different influences on the viscosity of these melts. The F2O-1
substitution causes a drastic decrease in viscosity over the entire investigated
range whereas the Cl2O-1 substitution causes a much
smaller decrease in viscosity in the high viscosity range and a slight
increase in viscosity in the low viscosity range (Fig. 3.6-8). As a consequence,
minor to major element abundances of Cl in strongly peralkaline undersaturated
volcanic rocks are not likely to have a significant influence on melt viscosity.
 |
 log10 log10 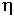 (Pa s) at comparison temperatures of 470, 570 and 1070°C. (Pa s) at comparison temperatures of 470, 570 and 1070°C. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page