

Zr and Hf have the same charge and similar ionic radii; consequently the geochemical behaviour of these elements is similar for a variety of different geological processes i.e, for many suites of igneous rocks the Zr/Hf ratio remains nearly constant with magmatic evolution. By contrast in other suites, peraluminous granites in particular, the Zr/Hf ratio decreases with fractionation, as does the Zr/Hf ratio of zircon-hafnon. The solubilities of zircon (ZrSiO4) and hafnon (HfSiO4) were therefore determined for water-saturated haplogranitic melts with peraluminous to peralkaline compositions in order to constrain the interpretation of the distribution of Zr-Hf in igneous environments.
Zircon and hafnon solubilities at 1035°C, 2 kbar and
H2O-saturation conditions increase dramatically with the alkali
content of the melt: from 0.25 wt% to 6.9 wt% HfO2 and from
0.09 wt% to 4.5 wt% ZrO2 for Al/(Na+K) compositions of 1.2 to
0.6, respectively. The mole percent of SiO2 of the melts is
constant, thus the activity of SiO2 in peralkaline to peraluminous
compositions is nearly constant, and the increases in zircon and hafnon
solubilities with alkali content is largely a consequence of decreases
of Zr and Hf activity coefficients. Futhermore, for a given Al/(Na+K) ratio,
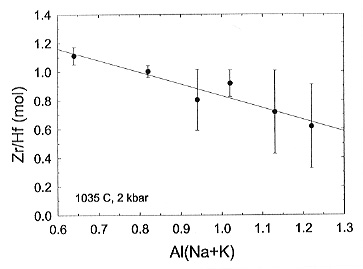
the division of the Zr and Hf molar abundances gives a value that is inversely
proportional to the Zr/Hf activity coefficient ratio. The molar Zr/Hf solubility
ratio is shown on Fig. 3.6-9. For strongly peralkaline
 |
|
[Al/(Na+K)=0.6 to 0.8] compositions this ratio is close to one. It follows that the Zr and Hf activity coefficients are similar and that the fractionation of alkaline granitic liquids should not result in a significant change of the Zr/Hf ratio. This agrees well with the natural data. In general the Zr/Hf ratio is also nearly constant for suites of and basaltic rocks, and thus it can be inferred that the Zr/Hf activity coefficient ratio of mafic melts is also near unity. By contrast the Zr/Hf solubility ratio is less than one for highly polymerised metaluminous [Al/(Na+K)=1.0] and peraluminous [Al/(Na+K)=1.2] melt composition, indicating that the Zr/Hf activity coefficient ratio is greater than one. This explains why for such melt compositions there are systematic decreases in the Zr/Hf ratio of zircon and of whole-rock values with increasing fractionation.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page