

Tantalum mineralization invariably is associated with granites and pegmatites
that are rich in lithium. This association potentially is explained, at
least in part, by chemical interactions between Li and Ta in melts. The
effect of lithium on the solubility of tantalite (MnTa2O6)
in water-saturated granitic melts was therefore experimentally determined
at 750° to 1035°C and 2 kbar. High concentrations of Nb, Zr, Hf
and W are also common in rare-metal granites and pegmatites, hence the
effect of Li on the solubilities of columbite (MnNb2O6),
zircon (ZrSiO4), hafnon (HfSiO4) and wolframite (MnWO4)
in granitic melts were also investigated. The haplogranitic melt compositions
used in these experiments have constant mole percent Si and Al, a constant
Na/K ratio, and the Al/(Li+Na+K) ratio was 1.0 for all experiments, i.e.,
the lithium content was the only compositional variable. Columbite and
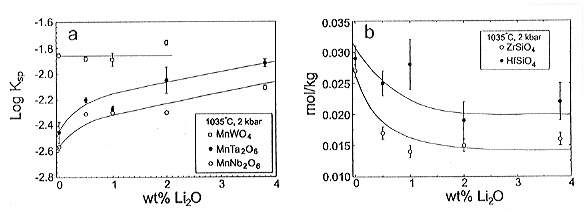
tantalite solubilities in water-saturated granitic liquids increase by
a factor of ~2 to 3 with the addition of 2 wt% Li2O (Fig. 3.6-10a).
By contrast, the lithium content of the melt does not affect the solubility
of wolframite and the solubilities of zircon and hafnon decrease with increasing
Li content of the melt (Fig. 3.6-10b). This most likely reflects the lower
field strength of Zr4+ and Hf4+,
 |
|
compared to Nb5+ and Ta+5, and consequently Zr and Hf are less able to compete with Li for non-bridging oxygens. The lack of dependence of wolframite solubility on Li content is consistent with W having a 6+ valence and behaving as a network former.
It is unlikely that natural melts are saturated with columbite-tantalite at 750°C, but this temperature is higher than the crystallization temperature of highly evolved granites. The solubility data can be extrapolated to 600°C, a more reasonable temperature, then be compared to abundances of Li-F-Mn-Fe-Ta-Nb in natural rocks and glasses that are representative of the liquids from which rare-metal granites and pegmatites crystallize. It is estimated that at 600°C these melt compositions could be saturated in columbite, but not tantalite. By contrast, tantalite saturation is predicted if the melts contained less Li and F. A potential explanation of the genesis of tantalum mineralization is that Ta is retained in the melt because of high Li-F concentrations. Tantalite crystallization is delayed until a Li±F±P mineral crystallizes, which lowers tantalite solubility, and results in a general association of Ta with Li.

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page