

Re is one of the HSE, which has special geochemical importance due to increasing interest in the Re/Os isotopic system. Little is known about Re solubility and partitioning behavior between metal and haplobasaltic silicate melts even at 1 atmosphere. We performed 1 atmosphere stirred crucible experiments on Re solubility which lasted 14 months and the final results from LA-ICP-MS measurements, performed in Newfoundland this year, highlight the difficulty of the experiments and their analysis.
Experiments were performed by equilibrating an anorthite-diopside eutectic composition melt with its containing vessel, a high-purity Re crucible, at a fixed temperature of 1400°C in a vertical gas mixing furnace. To accelerate the attainment of equilibrium the mechanically assisted equilibration technique was employed where the melt is permanently stirred with a high-purity Re spindle. The investigated oxygen fugacities were between -7 and -11 log units.
The attainment of equilibrium was directly monitored by time-series sampling at each fo2 condition. Samples were taken by dipping an Al2O3 rod into the melt, retracting the congealed melt droplet and quenching it in distilled water. The major element composition of the quenched glasses was determined by electron microprobe, while the trace element concentrations of Re were in a first attempt determined by instrumental neutron activation analyses (INAA), performed at the Max-Planck-Institute, Mainz. Samples were handpicked and checked for any obvious contamination from ReO2, which is volatile and can condense to form layers of ReO2 at any colder surface within the furnace. This causes a persistent hazard for sample contamination.
The results of the initial INAA measurements are given in Figure 3.3-6 and show a wide data scatter with apparently no dependence of the Re solubility on applied fo2. It became obvious that there was a problem with contamination and further investigation lead to the discovery of the so called "micro-nugget" effect: microscopically small clusters of metal contaminating the charges. To avoid or reduce this problem, a micro-analytical technique using just a tiny area of the sample for determining the "real" equilibrium concentration of Re is a clear advantage.
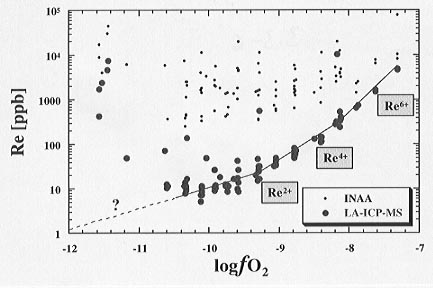 |
Fig. 3.3-6: Raw results of INAA (small closed circles) and LA-ICP-MS (big filled circles) measurements of 92 Re containing glass samples plotted versus the fo2 at which these samples were equilibrated. Curve fits for Re2+, Re4+, and Re6+ are supplied in the corresponding fO2 regimes. |
All 92 samples were measured in 2 successive days at Memorial University (Newfoundland). Figure 3.3-6 shows raw results from INAA and LA-ICP-MS measurements performed on identical experimental samples. The INAA results show scatter over nearly 2 orders of magnitude for a single fo2 condition, because micro-nuggets cannot be excluded from the bulk sample analysis. LA-ICP-MS results, on the other hand, show very little scatter because smaller areas of glass can be analysed and micro-nuggets avoided. In Figure 3.3-7 final results obtained from LA-ICP-MS measurements of the Re containing glasses are shown. At low fo2 Re is present in the melt as Re2+ but with increasing oxidation it passes through a mixed Re2+-Re4+ valence region and is finally present as Re6+ at high fo2. This clear trend is an impressive success in comparison to the previous INAA results and is both a warning as well as a clear indication that in trace element chemistry the analytical evaluation of the experiments and the choice of the optimum technique are crucial factors.
 |
Fig. 3.3-7: Re equilibrium solubilities versus applied fo2. The formal oxidation states determined from the slopes of the data in corresponding fo2 regimes are indicated. |

Tel: +49-(0) 921 55 3700 / 3766, Fax: +49-(0) 921 55 3769, E-mail: bayerisches.geoinstitut(at)uni-bayreuth.de
 Previous page
Previous page